Respiratory Pathogens Combined
Product name
HWTS-RT158A Respiratory Pathogens Combined Detection Kit(Fluorescence PCR)
Certificate
CE
Epidemiology
Corona Virus Disease 2019, referred to as ‘COVID-19’, refers to the pneumonia caused by 2019-nCoV infection. 2019-nCoV is a coronavirus belonging to the β genus. COVID-19 is an acute respiratory infectious disease, and the population is generally susceptible. At present, the source of infection is mainly patients infected by 2019-nCoV, and asymptomatic infected persons may also become the source of infection. Based on the current epidemiological investigation, the incubation period is 1-14 days, mostly 3-7 days. Fever, dry cough and fatigue are the main manifestations. A few patients had symptoms such as nasal congestion, runny nose, sore throat, myalgia and diarrhea, etc.
Influenza, commonly known as "flu", is an acute respiratory infectious disease caused by influenza virus. It is highly infectious. It is mainly transmitted by coughing and sneezing. It usually breaks out in spring and winter. Influenza viruses are divided into influenza A (IFV A), influenza B (IFV B), and Influenza C (IFV C) three types, all belong to sticky virus, cause human disease mainly for influenza A and B viruses, it is a single-stranded, segmented RNA virus. Influenza A virus is an acute respiratory infection, including H1N1, H3N2 and other subtypes, which are prone to mutation and outbreak worldwide. "Shift" refers to the mutation of influenza A virus, resulting in the emergence of a new virus "subtype". Influenza B viruses are divided into two lineages, Yamagata and Victoria. Influenza B virus only has antigenic drift, and it evades the human immune system's surveillance and elimination through its mutation. However, the evolution speed of influenza B virus is slower than that of human influenza A virus. Influenza B virus can also cause human respiratory infections and lead to epidemics.
Respiratory syncytial virus (RSV) is an RNA virus, belonging to the paramyxoviridae family. It is transmitted by air droplets and close contact and is the main pathogen of lower respiratory tract infection in infants. Infants infected with RSV may develop severe bronchiolitis and pneumonia, which are related to asthma in children. Infants have severe symptoms, including high fever, rhinitis, pharyngitis and laryngitis, and then bronchiolitis and pneumonia. A few sick children can be complicated with otitis media, pleurisy and myocarditis, etc. Upper respiratory tract infection is the main symptom of infection in adults and older children.
Channel
| FAM | SARS-CoV-2 |
| VIC(HEX) | RSV |
| CY5 | IFV A |
|
ROX |
IFV B |
|
Quasar 705 |
Internal Control |
Technical Parameters
| Storage |
-18℃ |
| Shelf-life | 12 months |
| Specimen Type | Oropharyngeal swab |
| Ct | ≤38 |
| LoD | 2019-nCoV: 300Copies/mL
Influenza A virus/Influenza B virus/Respiratory syncytial virus: 500Copies/mL |
| Specificity | a) Cross-reactivity results show that there is no cross reaction between the kit and human coronavirus SARSr-CoV, MERSr-CoV, HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63, parainfluenza virus type 1, 2, 3, rhinovirus A, B, C, chlamydia pneumoniae, human metapneumovirus, enterovirus A, B, C, D, epstein-barr virus, measles virus, human cytomegalo virus, rotavirus, norovirus, parotitis virus, varicella-zoster virus, legionella, bordetella pertussis, haemophilus influenzae, staphylococcus aureus, streptococcus pneumoniae, streptococcus pyogenes, klebsiella pneumoniae, mycobacterium tuberculosis, smoke aspergillus, candida albicans, candida glabrata, pneumocystis jiroveci and newborn cryptococcus and human genomic nucleic acid.
b) Anti-interference ability: select mucin (60mg/mL), 10% (v/v) of blood and phenylephrine (2mg/mL), oxymetazoline (2mg/mL), sodium chloride (including preservatives) (20mg/mL), beclomethasone (20mg/mL), dexamethasone (20mg/mL), flunisolide (20μg/mL), triamcinolone acetonide (2mg/mL), budesonide (2mg/mL), mometasone (2mg/mL), fluticasone (2mg/mL), histamine hydrochloride (5mg/mL), alpha interferon (800IU/mL), zanamivir (20mg/mL), ribavirin (10mg/mL), oseltamivir (60ng/mL), peramivir (1mg/mL), lopinavir(500mg/mL), ritonavir(60mg/mL), mupirocin (20mg/mL), azithromycin (1mg/mL), ceftriaxone (40μg/mL), meropenem (200mg/mL), levofloxacin (10μg/mL) and tobramycin (0.6mg/mL) for interference test, and the results show that interfering substances with concentrations mentioned above have no interference reaction to the test results of pathogens. |
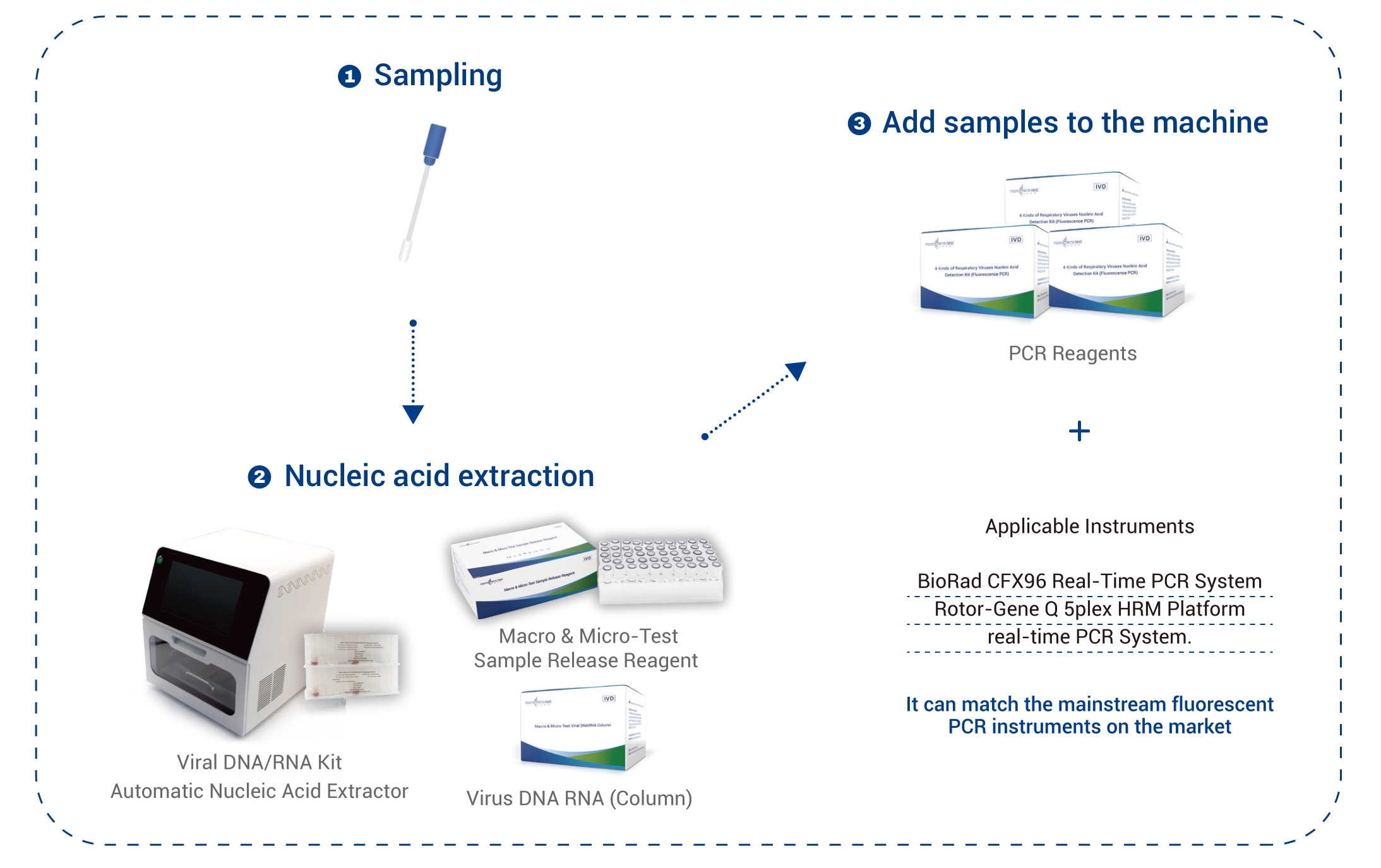
| Applicable Instruments | BioRad CFX96 Real-Time PCR System
Rotor-Gene Q 5plex HRM Platform real-time PCR System |