SARS-CoV-2 Virus Antigen – Home test
Product name
HWTS-RT062IA/B/C-SARS-CoV-2 Virus Antigen Detection Kit (colloidal gold method)-Nasal
Certificate
CE1434
Epidemiology
Coronavirus Disease 2019(COVID-19), is a pneumonia caused by infection with a novel coronavirus named as Severe Acute Respiratory Syndrome Corona-Virus 2 (SARS-CoV-2). SARS-CoV-2 is a novel coronavirus in β genus, enveloped particles in round or oval, with a diameter from 60 nm to 140 nm. Human is generally susceptible to SARS-CoV-2. The main sources of infection are the confirmed COVID-19 patients and asymptomatic carrier of SARSCoV-2.
Clinical study
The performance of Antigen Detection Kit was evaluated in 554 patients of nasal swabs collected from symptomatic suspects of COVID-19 within 7 days post symptom onset compared to RT-PCR assay. The performance of the SARS-CoV-2 Ag Test Kit is as follows:
| SARS-CoV-2 Virus Antigen (investigational reagent) | RT-PCR reagent | Total | |
| Positive | Negative | ||
| Positive | 97 | 0 | 97 |
| Negative | 7 | 450 | 457 |
| Total | 104 | 450 | 554 |
| Sensitivity | 93.27% | 95.0% CI | 86.62% - 97.25% |
| Specificity | 100.00% | 95.0% CI | 99.18% - 100.00% |
| Total | 98.74% | 95.0% CI | 97.41% - 99.49% |
Technical Parameters
| Storage temperature | 4℃-30℃ |
| Sample type | Nasal swab samples |
| Shelf life | 24 months |
| Auxiliary instruments | Not required |
| Extra Consumables | Not required |
| Detection time | 15-20 mins |
| Specificity | There is no cross-reactivity with pathogens such as human Coronavirus ( HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63), Novel influenza A H1N1 (2009), seasonal influenza A (H1N1, H3N2, H5N1, H7N9), Influenza B (Yamagata, Victoria), Respiratory syncytial virus A/B, Parainfluenza virus(1, 2 and 3), Rhinovirus (A, B, C), Adenovirus (1, 2, 3, 4,5, 7, 55). |
Work Flow
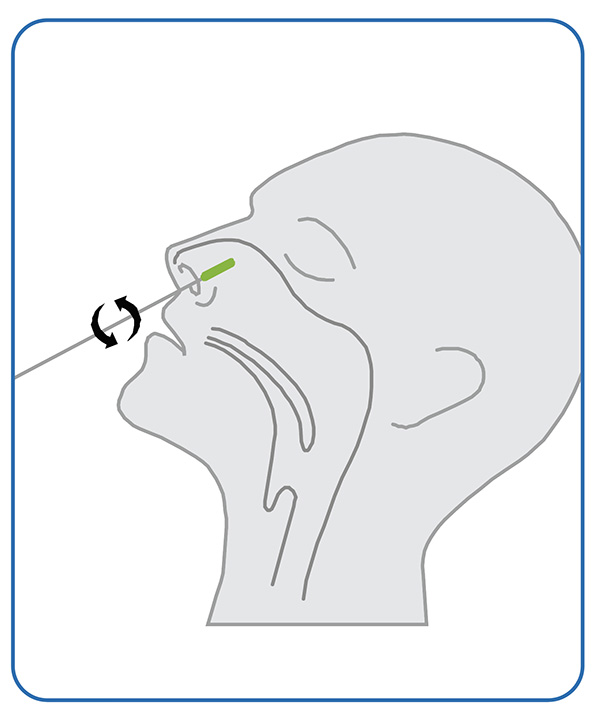
1. Sampling
● Gently insert the entire soft tip of the swab (usually 1/2 to 3/4 of an inch) into one nostril, Using medium pressure, rub the swab against all the inside walls of your nostril. Make at least 5 big circles. And each nostril must be swabbed for about 15 seconds.Using the same swab, repeat the same in your other nostril.
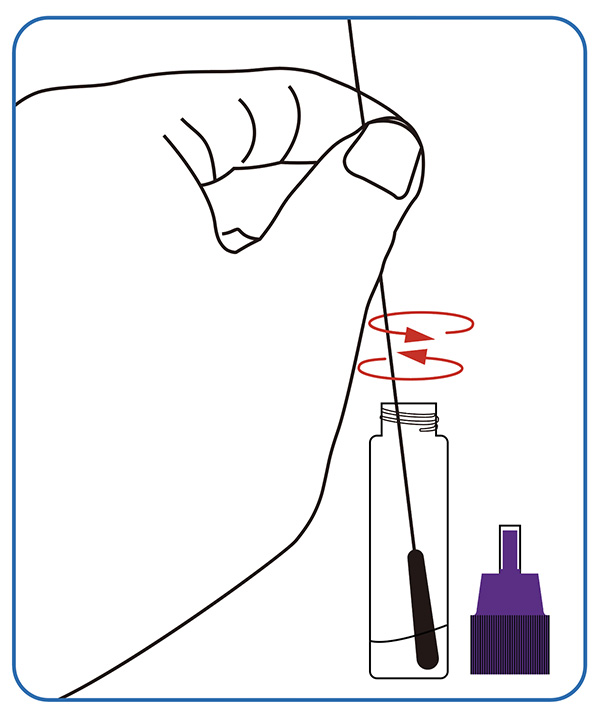
● Sample dissolving. Dip the swab completely into the sample extraction solution; Break the swab stick at the breaking point, leaving the soft end in the tube. Screw on the cap, invert 10 times and put the tube at a stable place.
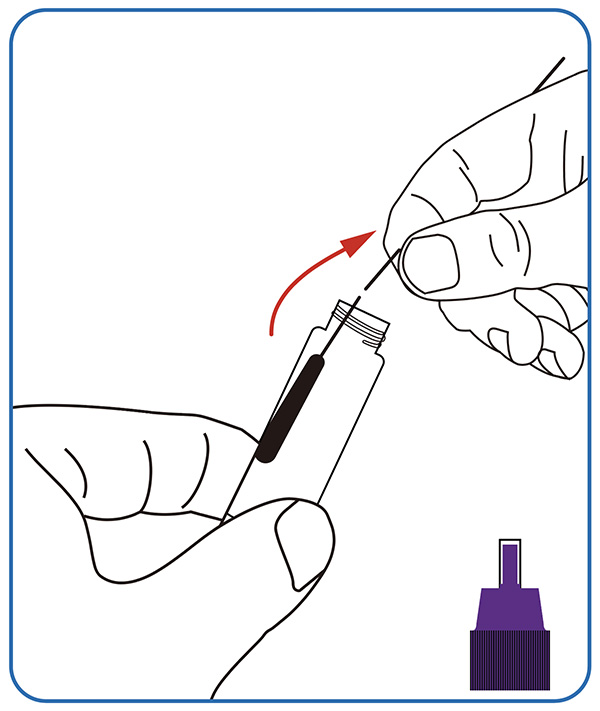
2. Perform the test
Put 3 drops of the processed extracted sample into the sample hole of the detection card, screw the cap.
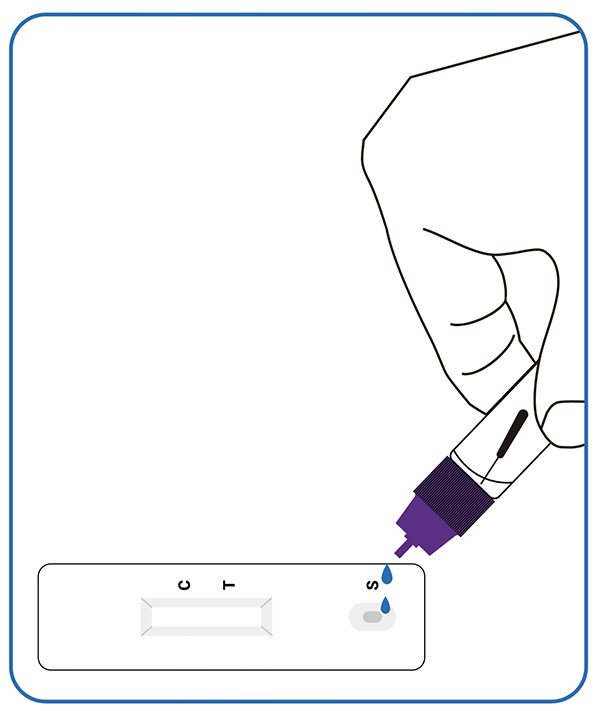
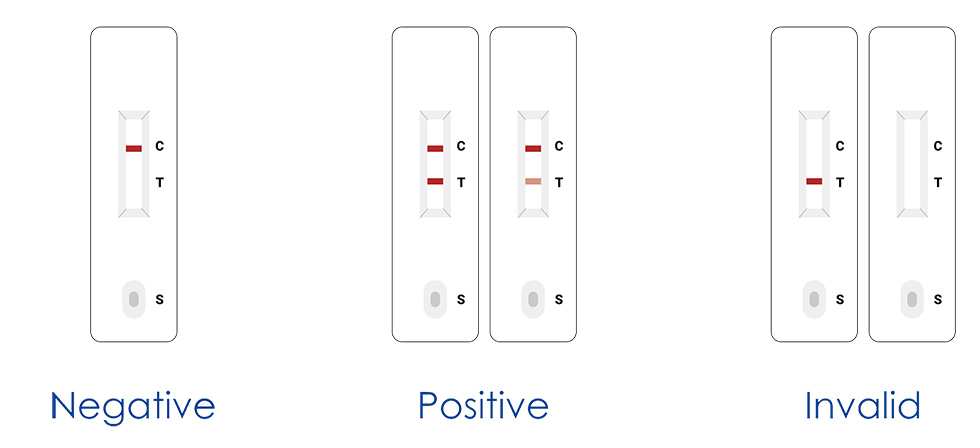
3. Read the result (15-20mins)