Best quality Malaria Pf Pv - Malaria Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test
Best quality Malaria Pf Pv - Malaria Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test Detail:
Product name
Plasmodium Nucleic Acid Detection Kit(Fluorescence PCR)
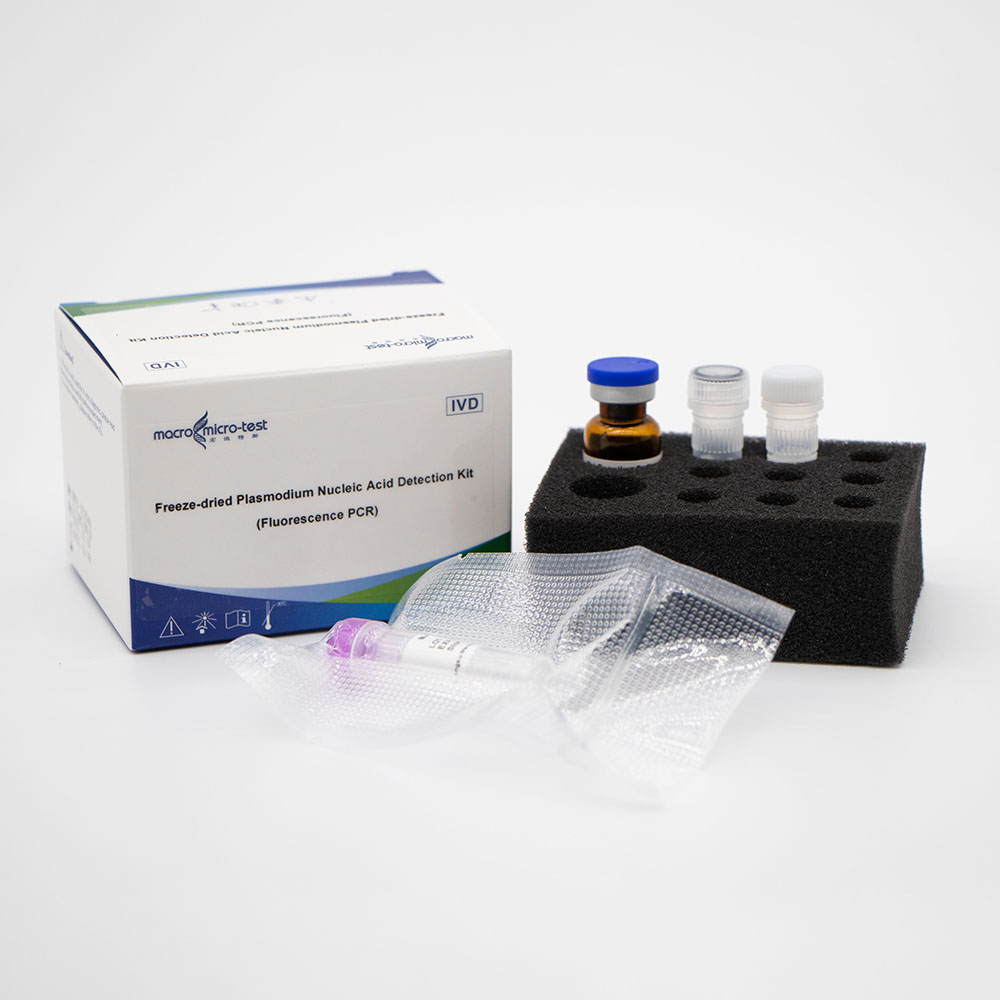
Freeze-dried Plasmodium Nucleic Acid Detection Kit (Fluorescence PCR)
Certificate
CE
Epidemiology
Malaria (Mal for short) is caused by Plasmodium, which is a single-celled eukaryotic organism, including Plasmodium falciparum Welch, Plasmodium vivax Grassi & Feletti, Plasmodium malariae Laveran, and Plasmodium ovale Stephens. It is a mosquito-borne and blood-borne parasitic disease that seriously endangers human health.
Of the parasites that cause malaria in humans, Plasmodium falciparum Welch is the deadliest. The incubation period of different malaria parasites is different, the shortest is 12-30 days, and the longer one can reach about 1 year. After the paroxysm of malaria, symptoms such as chills and fever may appear. The patients may have anemia and splenomegaly. The serious patients may have coma, severe anemia, acute renal failure which may lead to the death of patients[1]. Malaria is distributed worldwide, mainly in tropical and subtropical regions such as Africa, Central America, and South America[2].
Features
● Multiplex PCR Amplification Technology.
● Internal control: Fully monitor the experimental process to ensure the quality of Experiments.
● High specificity: No cross-reactivity with common respiratory pathogens for more accurate results.
● High sensitivity: 5 Copies/μL.
● Two options: lyophilized product & liquid product.
Channel
FAM: Plasmodium nucleic acid
VIC (HEX): Internal control
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 2 mins | No |
| 2 | 1 cycle | 95℃ | 5 mins | No |
| 3 | 40 cycles | 95℃ | 10 s | No |
| 58℃ | 30 s | Yes |
Technical Parameters
| Storage | ≤-18℃ in dark |
| Shelf-life | 24 months |
| Specimen Type | Whole blood, dried blood spots |
| Ct | ≤38 |
| CV | ≤5.0% |
| LoD | 5Copies/μL |
| Repeatability | Detect the company repeatability reference and calculate the coefficient of variation CV of Plasmodium detection Ct and the result≤ 5% (n=10). |
| Specificity | No cross reactivity with influenza A H1N1 virus, H3N2 influenza virus, influenza B virus, dengue fever virus, encephalitis B virus, respiratory syncytial virus, meningococcus, parainfluenza virus, rhinovirus, toxic bacillary dysentery, staphylococcus aureus, escherichia coli, streptococcus pneumoniae or klebsiella pneumoniae, salmonella typhi, and rickettsia tsutsugamushi, and the test results are all negative. |
| Applicable Instruments | It can match the mainstream fluorescent PCR instruments on the market. |
| SLAN-96P Real-Time PCR Systems | |
| ABI 7500 Real-Time PCR Systems | |
| ABI 7500 Fast Real-Time PCR Systems | |
| QuantStudio®5 Real-Time PCR Systems | |
| LightCycler®480 Real-Time PCR Systems | |
| LineGene 9600 Plus Real-Time PCR Detection Systems | |
| MA-6000 Real-Time Quantitative Thermal Cycler | |
| BioRad CFX96 Real-Time PCR System | |
| BioRad CFX Opus 96 Real-Time PCR System | |
Main Components
Plasmodium Nucleic Acid Detection Kit(Fluorescence PCR)
| Component |
Catalogue Number |
Quantity | Component Description | |
| HWTS-OT074A | HWTS-OT074B | |||
| Specification(20 tests/kit) | Specification(50 tests/kit) | |||
| Mal Reaction Buffer | 400μL/vial | 1 mL/vial | 1 vial | Contains Plasmodium, internal control specific primers, fluorescent probes, Taq enzyme, UDG enzyme, reaction buffer, etc. |
| Mal Positive Control | 400μL/vial | 1 mL/vial | 1 vial | Mixture of Plasmodium and internal control template |
| Mal Blank Control | 400μL/vial | 1 mL/vial | 1 vial | DNase/RNase free H2O |
Freeze-dried Plasmodium Nucleic Acid Detection Kit (Fluorescence PCR)
| Component | Character |
Catalogue Number |
Component Description | |
| HWTS-OT054A | HWTS-OT054B | |||
| Specification/Quantity(20 tests/kit) | Specification/Quantity (50 tests/kit) | |||
| Mal Reaction Buffer | lyophilized | 1 bottle | 1 bottle | Contains Plasmodium, internal control specific primers, fluorescent probes, Taq enzyme, UDG enzyme, reaction buffer, etc. |
| Mal Positive Control | lyophilized | 1 vial | 1 vial | Mixture of Plasmodium and internal control template |
| Mal Blank Control | Liquid | 1 vial, 600μL/vial | 1 vial, 600μL/vial | DNase/RNase free H2O |
| Reconstituted Solution | Liquid | 1 vial, 1mL/vial | 1 vial, 1.45mL/vial | DNase/RNase free H2O, etc. |
References
[1] Organization W H. World Malaria Report 2014 (PDF)[J]. 2015.
[2] Zwaenepoel B, Jan Philippé, Callens S. Screening naar malaria: LAMP-test als sensitiever alternatief voor de dikdruppeltest en implementatie hiervan in het UZ Gent[J]. Tijdschrift voor Geneeskunde, 2019, 75:914.
Product detail pictures:



Related Product Guide:
It really is our obligation to satisfy your requirements and efficiently serve you. Your fulfillment is our greatest reward. We're hunting forward to your check out for joint development for Best quality Malaria Pf Pv - Malaria Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test , The product will supply to all over the world, such as: London, Sudan, Luxembourg, Regarding quality as survival, prestige as guarantee, innovation as motive force, development along with advanced technology, our group hopes to make progress together with you and make untiring efforts for the bright future of this industry.
The company has a good reputation in this industry, and finally it tured out that choose them is a good choice.