Top Suppliers Ebv Pcr Test - EB Virus Nucleic Acid Detection Kit(Fluorescence PCR) – Macro & Micro-Test
Top Suppliers Ebv Pcr Test - EB Virus Nucleic Acid Detection Kit(Fluorescence PCR) – Macro & Micro-Test Detail:
Product name
EB Virus Nucleic Acid Detection Kit(Fluorescence PCR)
Certificate
CE
Epidemiology
EBV (Epstein-barr virus), or human herpesvirus type 4, is a common human herpesvirus[1]. In recent years, a large number of studies have proved that EBV is associated with occurrence and development of nasopharyngeal cancer, Hodgkin’s disease, T/Natural killer celllymphoma, Burkitt’s lymphoma, breast cancer, gastric cancer and other malignant tumors. And it is also closely associated with post-transplantlymphoproliferative disorders, post-transplant smooth muscle tumor and acquired immunedeficiency syndrome(AIDS) related lymphoma, multiple sclerosis, primary central nervous system lymphoma or leiomyosarcoma[2-4].
Features
● Multiplex PCR Amplification Technology.
● Internal control: Fully monitor the experimental process to ensure the quality of Experiments.
● High sensitivity: 500Copies/mL.
● Convenient: One test for multiple results.
Channel
FAM: EBV
VIC (HEX): Internal control
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 2mins | No |
| 2 | 1 cycle | 95℃ | 5mins | No |
| 3 | 40 cycles | 95℃ | 15s | No |
| 58℃ | 30s | Yes |
Technical Parameters
| Storage | ≤-18℃ In dark |
| Shelf-life | 12 months |
| Specimen Type | Whole blood, Plasma, Serum |
| Ct | ≤38 |
| CV | ≤5.0% |
| LoD | 500Copies/mL |
Specificity: It has no cross-reactivity with other pathogens (such as human herpesvirus 1, 2, 3, 6, 7, 8, hepatitis B virus, cytomegalovirus, influenza A, etc.) or bacteria (Staphylococcus aureus, Candida albicans, etc.)
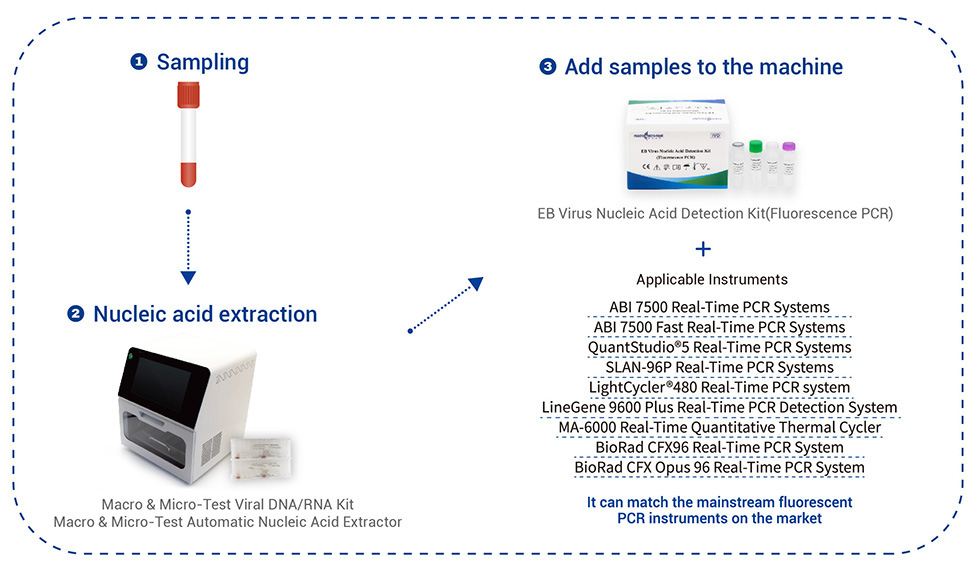
Applicable Instruments: It can match the mainstream fluorescent PCR instruments on the market.
SLAN-96P Real-Time PCR Systems.
ABI 7500 Real-Time PCR Systems.
QuantStudio®5 Real-Time PCR Systems.
LightCycler®480 Real-Time PCR Systems.
LineGene 9600 Plus Real-Time PCR Detection Systems.
MA-6000 Real-Time Quantitative Thermal Cycler.
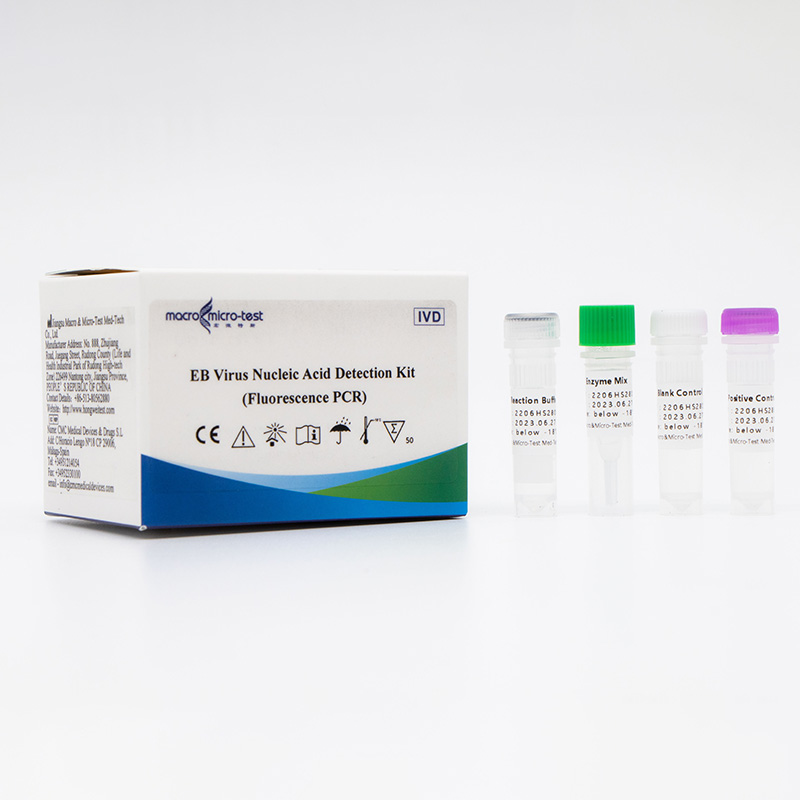
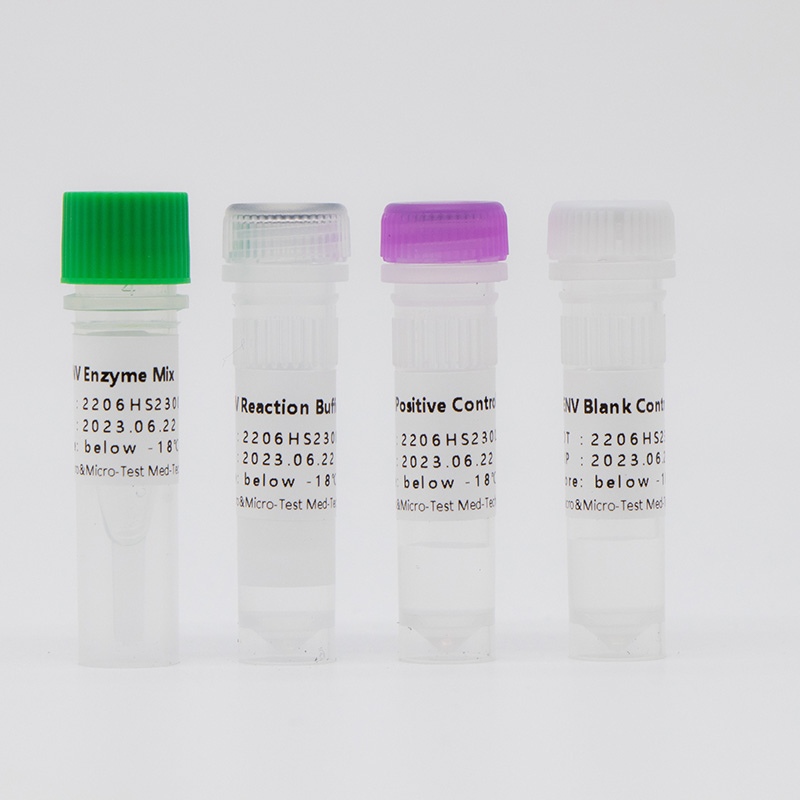
Main Components
| Catalogue Number | Component (50 tests/kit) | Specification | Quantity | Component Description |
| HWTS-OT061A | EB Reaction Buffer | 1.175mL/vial | 1 vial | Amplification reaction reagents, EB virus primer probes, internal reference primer probes, etc. |
| EB Enzyme Mix | 75μL/vial | 1 vial | Taq enzyme, UDG enzyme, etc. | |
| EB Positive Control | 600μL/vial | 1 vial | Mixed solution of viral target gene and internal reference | |
| EB Blank Control | 600μL/vial | 1 vial | DNase/RNase free H2O |
Total PCR Solution
Reference
[1] ohen JI, Jaffe ES, Dale JK, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States[J]. Blood, 2011, 117(22): 5835-5849.
[2] Jahangiryan A, Kheirandish M, Samiee S, et al. Determination of Epstein-Barr virus (EBV) incidence in umbilical cord blood (UCB) and assessment of virus DNA via Real-time PCR[J]. Annals of Cancer Research and Therapy, 2021, 29(1):114-120.
[3] Petersson F. Nasopharyngeal carcinoma:a review[J].Semin Diagn Pathol, 2015, 32(1):54-73. [4]Chen J N, He D, Tang F, et al. Epstein-Barr virus-associated gastric carcinoma:a newly defined entity[J]. J Clin Gastroenterol, 2012, 46(4):262-271.
Product detail pictures:


Related Product Guide:
We've one of the most advanced generation tools, experienced and qualified engineers and workers, recognized good quality manage systems and a friendly skilled product sales workforce pre/after-sales support for Top Suppliers Ebv Pcr Test - EB Virus Nucleic Acid Detection Kit(Fluorescence PCR) – Macro & Micro-Test , The product will supply to all over the world, such as: Tajikistan, Auckland, Latvia, As the world economic integration bringing challenges and opportunities to the xxx industry, our company , by carrying on our teamwork, quality first, innovation and mutual benefit, are confident enough to supply our clients sincerely with qualified merchandise, competitive price and great service, and to build a brighter future under the spirit of higher, faster, stronger with our friends together by carrying on our discipline.
Good quality and fast delivery, it's very nice. Some products have a little bit problem, but the supplier replaced timely, overall, we are satisfied.


