Renewable Design for Dengue Detection Kit - Dengue Virus IgM/IgG Antibody Detection Kit (Immunochromatography) – Macro & Micro-Test
Renewable Design for Dengue Detection Kit - Dengue Virus IgM/IgG Antibody Detection Kit (Immunochromatography) – Macro & Micro-Test Detail:
Product name
Dengue Virus IgM/IgG Antibody Detection Kit (Immunochromatography)
Certificate
CE
Epidemiology
Dengue fever is an acute infectious disease caused by dengue virus, and it is also one of the most widely spread mosquito-borne infectious diseases in the world. Serologically, it is divided into four serotypes, DENV-1, DENV-2, DENV-3, and DENV-4[1]. Dengue virus can cause a series of clinical symptoms. Clinically, the main symptoms are sudden high fever, extensive bleeding, severe muscle pain and joint pain, extreme fatigue, etc., and are often accompanied by rash, lymphadenopathy and leukopenia[2]. With the increasingly serious global warming, the geographicaldistribution of dengue fever tends to spread, and the incidence and severity of the epidemic also increase. Dengue fever has become a serious global public health problem.
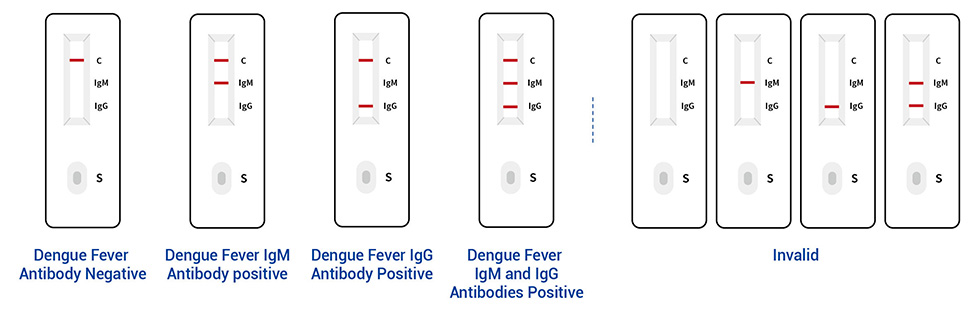
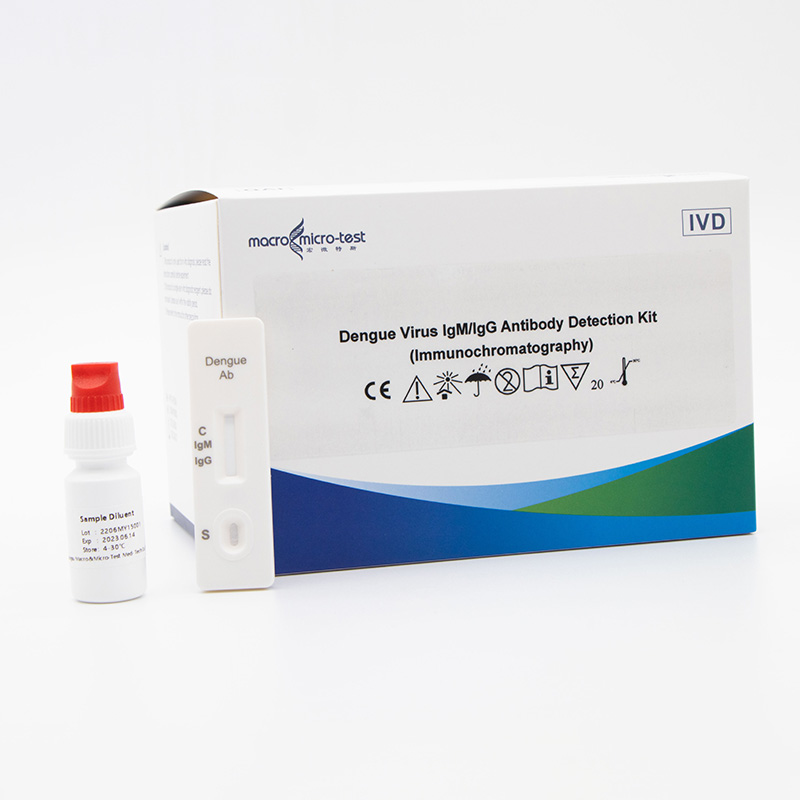
This product is a rapid, on-site and accurate detection kit for dengue virus antibody (IgM/IgG). If it is positive for IgM antibody, it indicates a recent infection. If it is positive for IgG antibody, it indicates a longer infection time or previous infection. In patients with primary infection, IgM antibodies can be detected 3-5 days after the onset, and peak after 2 weeks, and can be maintained for 2-3 months; IgG antibodies can be detected 1 week after the onset, and IgG antibodies can be maintained for several years or even whole life. Within 1 week, If the detection of a high level of specific IgG antibody in the serum of the patient within one week of the onset, it indicates a secondary infection, and a comprehensive judgment can also be made in combination with the ratio of IgM/IgG antibody detected by the capture method. This method can be used as a supplement to viral nucleic acid detection methods.
Features
● Rapid: Read results within 15 minutes
● Easy to use: Only 3 steps
● Convenient: No instrument
● Room temperature: Transportation & storage at 4-30℃ for 12 months
● Accuracy: High sensitivity & specificity
Technical Parameters
| Target region | Dengue IgM and IgG |
| Storage temperature | 4℃-30℃ |
| Sample type | Human serum, plasma, venous blood and peripheral blood |
| Shelf life | 12 months |
| Auxiliary instruments | Not required |
| Extra Consumables | Not required |
| Detection time | 15-20 mins |
| Specificity | There is no cross-reactivity with Japanese encephalitis virus, forest encephalitis virus, hemorrhagic fever with thrombocytopenia syndrome, Xinjiang hemorrhagic fever, Hantavirus, hepatitis C virus, influenza A virus, influenza B virus. |
Work Flow
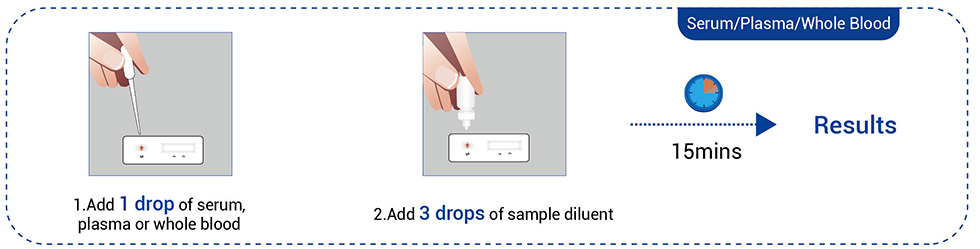
● Venous blood (Serum, Plasma, or Whole blood)
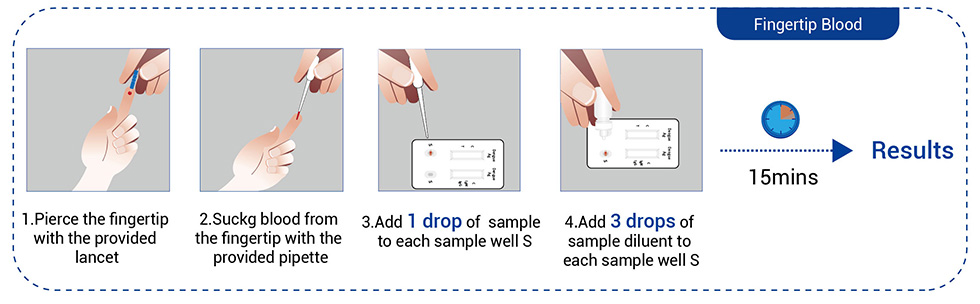
● Peripheral blood (Fingertip blood)
● Read the result (15-20 mins)
Precautions:
1. Do not read the result after 20 mins.
2. After opening, please use the product within 1 hour.
3. Please add samples and buffers in strict accordance with the instructions.
Product detail pictures:


Related Product Guide:
Our improvement depends on the superior equipment, excellent talents and continuously strengthened technology forces for Renewable Design for Dengue Detection Kit - Dengue Virus IgM/IgG Antibody Detection Kit (Immunochromatography) – Macro & Micro-Test , The product will supply to all over the world, such as: America, Rwanda, Sheffield, Our company sets up several departments, including production department, sales department, quality control department and sevice center,etc. only for accomplish the good-quality product to meet customer's demand, all of our products have been strictly inspected before shipment. We always think about the question on the side of the customers,because you win,we win!
High production efficiency and good product quality, fast delivery and completed after-sale protection, a right choice, a best choice.