Online Exporter Respiratory Pathogen Kit - RT-PCR kit for detecting six kinds of respiratory pathogens – Macro & Micro-Test
Online Exporter Respiratory Pathogen Kit - RT-PCR kit for detecting six kinds of respiratory pathogens – Macro & Micro-Test Detail:
Product name
Real time fluorescent RT-PCR kit for detecting six kinds of respiratory pathogens
Certificate
CE
Epidemiology
Corona Virus Disease 2019, referred to as “COVID-19″, refers to the pneumonia caused by SARS-CoV-2 infection. SARS-CoV-2 is a coronavirus belonging to the β genus. COVID-19 is an acute respiratory infectious disease, and the population is generally susceptible. At present, the source of infection is mainly patients infected by SARS-CoV-2, and asymptomatic infected persons may also become the source of infection. Based on the current epidemiological investigation, the incubation period is 1-14 days, mostly 3-7 days. Fever, dry cough and fatigue are the main manifestations. A few patients had nasal congestion, runny nose, sore throat, myalgia and diarrhea.
Influenza, commonly known as “flu”, is an acute respiratory infectious disease caused by influenza virus. It is highly infectious. It is mainly transmitted by coughing and sneezing. It usually breaks out in spring and winter. Influenza viruses are divided into influenza A, IFV A, influenza B, IFV B, and Influenza C, IFV C three types, all belong to sticky virus, cause human disease mainly for influenza A and B viruses, it is a single-stranded, segmented RNA virus. Influenza A virus is an acute respiratory infection, including H1N1, H3N2 and other subtypes, which are prone to mutation and outbreak worldwide. “Shift” refers to the mutation of influenza A virus, resulting in the emergence of a new virus “subtype”. Influenza B viruses are divided into two lineages, Yamagata and Victoria. Influenza B virus only has antigenic drift, and it evades the human immune system’s surveillance and elimination through its mutation. However, the evolution speed of influenza B virus is slower than that of human influenza A virus. Influenza B virus can also cause human respiratory infections and lead to epidemics.
Adenovirus (AdV) belongs to mammalian adenovirus, which is a double stranded DNA virus without envelope. At least 90 genotypes have been found, which can be divided into A-G 7 subgenera. AdV infection can cause a variety of diseases, including pneumonia, bronchitis, cystitis, eye conjunctivitis, gastrointestinal diseases and encephalitis. Adenovirus pneumonia is one of the more severe types of community-acquired pneumonia in children, accounting for about 4%-10% of community-acquired pneumonia.
Mycoplasma pneumoniae (MP) is a kind of the smallest prokaryotic microorganism, which is between bacteria and virus, with cell structure but no cell wall. MP mainly causes human respiratory tract infection, especially in children and young people. It can cause human mycoplasma pneumonia, children’s respiratory tract infection and atypical pneumonia. The clinical manifestations are various, most of which are severe cough, fever, chills, headache, sore throat. Upper respiratory tract infection and bronchial pneumonia are the most common. Some patients can develop from upper respiratory tract infection to severe pneumonia, severe respiratory distress and death may occur.
Respiratory syncytial virus (RSV) is an RNA virus, belonging to the paramyxoviridae family. It is transmitted by air droplets and close contact and is the main pathogen of lower respiratory tract infection in infants. Infants infected with RSV may develop severe bronchiolitis (referred to as bronchiolitis) and pneumonia, which are related to asthma in children. Infants have severe symptoms, including high fever, rhinitis, pharyngitis and laryngitis, and then bronchiolitis and pneumonia. A few sick children can be complicated with otitis media, pleurisy and myocarditis, etc. Upper respiratory tract infection is the main symptom of infection in adults and older children.
Features
● Multiplex PCR Amplification Technology.
● Internal control: Fully monitor the experimental process to ensure the quality of Experiments.
● High sensitivity: 300Copies/mL.
● Convenient: One test for multiple results.
● Comprehensive: Wide coverage of pathogen types, with detection of various subtypes available.
Channel
| The name of the channel | R6 Reaction Buffer A | R6 Reaction Buffer B |
| FAM | SARS-CoV-2 | HAdV |
| VIC/HEX | Internal Control | Internal Control |
| CY5 | IFV A | MP |
| ROX | IFV B | RSV |
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 5mins | No |
| 2 | 1 cycle | 95℃ | 1min | No |
| 3 | 40 cycles | 95℃ | 5s | No |
| 55℃ | 15s | Yes |
Technical Parameters
| Storage | ≤-18℃ In dark |
| Shelf-life | 9 months |
| Specimen Type | Whole blood, Plasma, Serum |
| Ct | ≤38 |
| CV | ≤5.0% |
| LoD | 300Copies/mL |
Specificity: Cross-reactivity results showed that there was no cross reaction between the kit and human coronavirus SARSr-CoV, MERSr-CoV, HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63, parainfluenza virus type 1, 2, 3, rhinovirus A, B, C, chlamydia pneumoniae, human metapneumovirus, enterovirus A, B, C, D, human pulmonary virus, epstein-barr virus, measles virus, human cytomegalo virus, rotavirus, norovirus, parotitis virus, varicella-zoster virus, legionella, bordetella pertussis, haemophilus influenzae, staphylococcus aureus, streptococcus pneumoniae, s. pyogenes, klebsiella pneumoniae, mycobacterium tuberculosis, smoke aspergillus, candida albicans, candida glabrata, pneumocystis jiroveci and newborn cryptococcus and human genomic nucleic acid.
Applicable Instruments: It can match the mainstream fluorescent PCR instruments on the market.
SLAN-96P Real-Time PCR Systems.
ABI 7500 Real-Time PCR Systems.
ABI 7500 Fast Real-Time PCR Systems.
QuantStudio®5 Real-Time PCR Systems.
LightCycler®480 Real-Time PCR Systems.
LineGene 9600 Plus Real-Time PCR Detection Systems.
MA-6000 Real-Time Quantitative Thermal Cycler.
BioRad CFX96 Real-Time PCR System, BioRad.
CFX Opus 96 Real-Time PCR System.
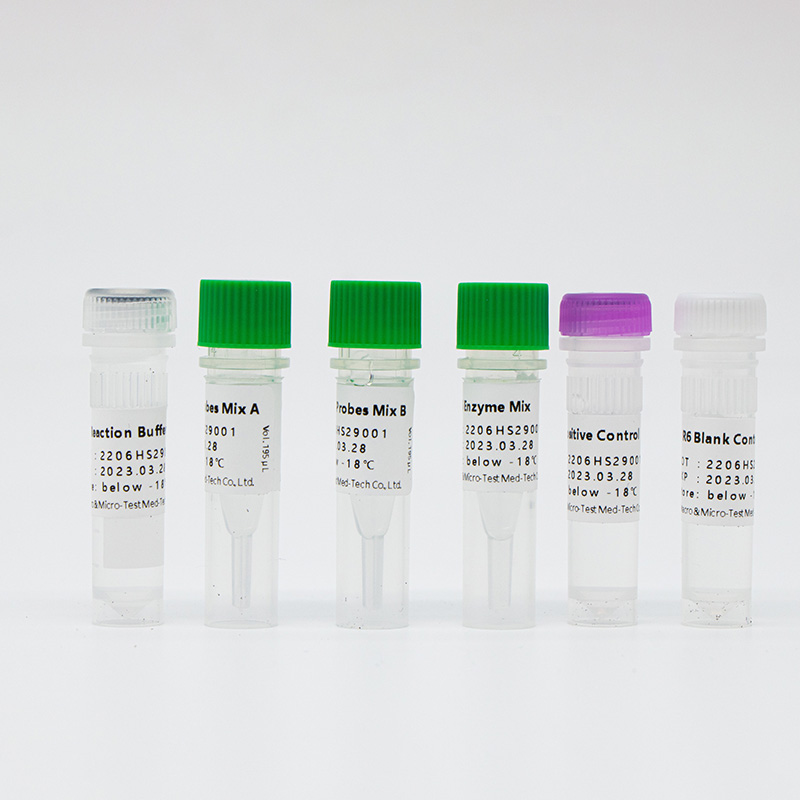
Main Components
Lyophilized product
| Component | Character | Catalogue Number | Component Description | ||
| HWTS-OT058B | HWTS-OT058C | HWTS-OT058Z | |||
| Specification/Quantity
(50 tests/kit) |
Specification/Quantity
(20 tests/kit) |
Specification/Quantity
(48 tests/kit) |
|||
| R6 Reaction Buffer A | lyophilized | 1 bottle | 1 bottle | 8-tube strips, 6 strips | Amplification reaction buffer, dNTP, specific primers and probes of pathogens including SARS-CoV-2, IFV A, IFV B and internal control, Taq enzyme, reverse transcriptase, UDG enzyme, etc. |
| R6 Reaction Buffer B | lyophilized | 1 bottle | 1 bottle | 8-tube strips, 6 strips | Amplification reaction buffer, dNTP, specific primers and probes of pathogens including AdV, MP, RSV and internal control, Taq enzyme, reverse transcriptase, UDG enzyme, etc. |
| R6 Positive Control | lyophilized | 1 vial | 1 vial | 1 vial | SARS-CoV-2, IFV A, IFV B ,AdV, MP, RSV gene plasmids and internal control plasmids mix |
| R6 Blank Control | Liquid | 1 vial, 600μL/vial | 1 vial, 600μL/vial | 1 vial, 600μL/vial | DNase/RNase free H2O |
| R6 Reconstituted Solution | Liquid | 2 vials, 1.1mL/vial | 1 vial, 1.3mL/vial | 2 vials, 1.07mL/vial | DNase/RNase free H2O, etc. |
| Catalogue Number | Component (50 tests/kit) | Specification | Quantity | Component Description |
| HWTS-OT058A | R6 ReactionBuffer | 1.6mL/vial | 1 vial | Amplification reaction buffer, dNTP |
| R6 Primers andProbes Mix A | 195μL/vial | 1 vial | Specific primers and probes of pathogens including SARS-CoV-2, IFV A, IFV B and internal control | |
| R6 Primers andProbes Mix B | 195μL/vial | 1 vial | Specific primers and probes of pathogens including AdV, MP, RSV and internal control | |
| R6 Enzyme Mix | 160μL/vial | 1 vial | Taq enzyme, reverse transcriptase, UDG enzyme | |
| R6 PositiveControl | 750μL/vial | 1 vial | Virus target gene plasmids and internal control plasmids mix | |
| R6 Blank Control | 750μL/vial | 1 vial | DNase/RNase free H2O |
Total PCR Solution

References
[1] Rai P, Kumar B K, Deekshit V K, et al. Detection technologies and recent developments in the diagnosis of COVID-19 infection[J]. Applied Microbiology and Biotechnology, 2021, 105(5).
[2] Marando M, Tamburello A, Gianella P. False-Negative Nasopharyngeal Swab RT-PCR Assays in Typical COVID-19: Role of Ultra-low-dose Chest CT and Bronchoscopy in Diagnosis[J]. European Journal of Case Reports in Internal Medicine, 2020, 7(7).
[3] Peiris J, Poon L, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans[J]. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology, 2009, 45(3):169-173.
[4] Yamashita M, Krystal M, Fitch W M, et al. Influenza B virus evolution: Co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses[J]. Virology, 1988, 163(1):112-122.
[5] Sloots T P, Whiley D M , Syrmis M W , et al. lightcycler reverse transcriptase pcr virus in respiratory samples by detection of human respiratory syncytial[J]. 2019.
[6] Yw A, Ek B, Ar B, et al. Comparison of the Idylla Respiratory (IFV-RSV) panel with the GeneXpert Xpert Flu/RSV assay: a retrospective study with nasopharyngeal and midturbinate samples[J]. Diagnostic Microbiology and Infectious Disease, 2019, 94( 1):33-37.
[7] Leong N K C, Chu D K W, Chu J T S, et al. A six﹑lex droplet digital RT ㏄ CR assay for seasonal influenza virus typing, subtyping, and lineage determination[J]. Influenza and Other Respiratory Viruses, 2020.
[8] Pol F, S Quéguiner, Gorin S, et al. Validation of commercial real-time RT-PCR kits for detection of influenza A viruses in porcine samples and differentiation of pandemic (H1N1) 2009 virus in pigs[J]. Journal of Virological Methods, 2011, 171(1):241-247.
[9] Coiras M T, Aguilar J C, M.L. García, et al. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays.[J]. Journal of Medical Virology, 2004, 72(3):484.
[10] Fre’ de’ric Raymond, Julie Carbonneau, Nancy Boucher et al. Comparison of Automated Microarray Detection with Real-Time PCR Assays for Detection of Respiratory Viruses in Specimens Obtained from Children [J]. Journal of Clinical Microbiology,2009, 47(3):740-750.
[11] Metzgar D, Gibbins C, Hudson N R, et al. Evaluation of multiplex type-specific real-time PCR assays using the LightCycler and joint biological agent identification and diagnostic system platforms for detection and quantitation of adult human respiratory adenoviruses.[J]. Journal of Clinical Microbiology, 2010, 48(4):1397-1403.
[12] Tatsumi C, Iizuka S, Mita T, et al. The first identification of human adenovirus 57 (HAdV-57) in Japan[J]. Japanese journal of infectious diseases, 2018, 71(4).
[13] Kanwar N , Hassan F , Nguyen A , et al. Head-to-head comparison of the diagnostic accuracies of BD Veritor System RSV and Quidel Sofia RSV FIA systems for respiratory syncytial virus (RSV) diagnosis.[J]. Journal of Clinical Virology, 2015, 65:83-86.
[14] Bing S , He H , Zheng W , et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study[J]. Critical Care, 2014, 18(4):456.
[15] CaoB, HuangGH, PuZH, et al.Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia[J].Chest, 2014, 145(1):79-86.
Product detail pictures:

Related Product Guide:
We insist on offering high quality production with good business concept, honest sales and the best and fast service. it will bring you not only the high quality product and huge profit, but the most significant is to occupy the endless market for Online Exporter Respiratory Pathogen Kit - RT-PCR kit for detecting six kinds of respiratory pathogens – Macro & Micro-Test , The product will supply to all over the world, such as: Peru, Germany, Haiti, We always adhere to follow the honesty, mutual benefit, common development, after years of development and the tireless efforts of all staff, now has perfect export system, diversified logistics solutions, thorough meet customer shipping, air transport, international express and logistics services. Elaborate one-stop sourcing platform for our customers!
The factory technical staff gave us a lot of good advice in the cooperation process, this is very good, we are very grateful.