OEM manufacturer Epstein-Barr Virus - Macro & Micro-Test Sample Release Reagent – Macro & Micro-Test
OEM manufacturer Epstein-Barr Virus - Macro & Micro-Test Sample Release Reagent – Macro & Micro-Test Detail:
Product name
Macro & Micro-Test Sample Release Reagent
Certificate
CE, FDA, NMPA
Features
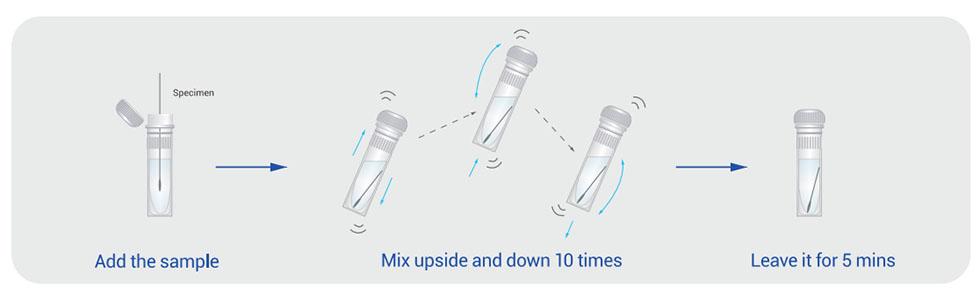
Rapid: only 5 mins.
Easy: only 3 steps (1.add the sample 2.Mix upside and down 10 times 3. Leave it for 5 mins).
Main components
| Name | Main components | Component specifications | Quantity |
| Sample Release reagent | Dithiothreitol, sodium dodecyl sulfate (SDS), RNase inhibitor, surfactant, purified water | 0.5mL/Vial | 50 Vial |
Note: Components in different batches of kits are not interchangeable.
Storage conditions and shelf life
Store and transport at room temperature. The shelf life is 24 months.
Applicable instruments
Instruments and equipment during sample processing, such as pipettes, vortex mixers, water baths, etc.
Sample requirements
Freshly collected oropharyngeal swabs, nasopharyngeal swabs.
Precision
When this kit is used for extraction from the in-house precision reference CV for 10 replicates, the coefficient of variation (CV, %) of Ct value is not more than 10%.
Inter-batch difference
When the in-house precision reference is tested on three batches of kits under trial production upon repeated extraction and, the coefficient of variation (CV, %) of Ct value is not more than 10%.
Performance comparison
● Extraction efficiency comparsion
|
Efficiency comparsion of magnetic beads method and sample releaser |
||||
|
concentration |
magnetic beads method |
sample releaser |
||
|
orfab |
N |
orfab |
N |
|
|
20000 |
28.01 |
28.76 |
28.6 |
29.15 |
|
2000 |
31.53 |
31.9 |
32.35 |
32.37 |
|
500 |
33.8 |
34 |
35.25 |
35.9 |
|
200 |
35.25 |
35.9 |
35.83 |
35.96 |
|
100 |
36.99 |
37.7 |
38.13 |
undet |
The extraction efficiency of sample releaser was similar to that of magnetic beads method, and the concentration of pathogen could be 200Copies/mL.
● CV value comparison
|
Repeatability of sample releaser extraction |
||
| concentration:5000Copies/mL |
ORF1ab |
N |
|
30.17 |
30.38 |
|
|
30.09 |
30.36 |
|
|
30.36 |
30.26 |
|
|
30.03 |
30.48 |
|
|
30.14 |
30.45 |
|
|
30.31 |
30.16 |
|
|
30.38 |
30.7 |
|
|
30.72 |
30.79 |
|
| CV |
0.73% |
0.69% |
When tested at 5,000 copies /mL, the CV of orFab and N was 0.73% and 0.69%, respectively.
Product detail pictures:


Related Product Guide:
We not only will try our greatest to supply outstanding services to every shopper, but also are ready to receive any suggestion offered by our buyers for OEM manufacturer Epstein-Barr Virus - Macro & Micro-Test Sample Release Reagent – Macro & Micro-Test , The product will supply to all over the world, such as: El Salvador, Zimbabwe, Stuttgart, Qualified R&D engineer might be there for your consultation service and we will try our best to meet your requirements. So you should feel free to contact us for inquiries. You'll be able to send us emails or call us for small business. Also you are able to come to our business by yourself to get further knowing of us. And we are going to surely present you with the best quotation and after-sale service. We're ready to build stable and friendly relations with our merchants. To achieve mutual success, we'll make our best efforts to build a solid co-operation and transparent communication work with our companions. Above all, we're here to welcome your inquiries for any of our merchandise and service.
The sales manager has a good English level and skilled professional knowledge, we have a good communication. He is a warm and cheerful man, we have a pleasant cooperation and we became very good friends in private.