Group B Streptococcus Nucleic Acid
Product Name
HWTS-UR010A-Nucleic Acid Detection Kit based on Enzymatic Probe Isothermal Amplification (EPIA) for Group B Streptococcus
Epidemiology
Group B Streptococcus (GBS), also known as streptococcus agalcatiae, is a gram-positive pathogen that normally resides in the lower digestive tract and urogenital tract of the human body. About 10%-30% of pregnant women have GBS vaginal residence. Pregnant women are susceptible to GBS due to the changes in internal environment of the reproductive tract caused by changes in hormone levels in the body, which can lead to adverse pregnancy outcomes such as premature delivery, premature rupture of membranes, and stillbirth, and can also lead to puerperal infections in pregnant women. In addition, 40%-70% of women infected with GBS will transmit GBS to their newborns during delivery through the birth canal, causing severe neonatal infectious diseases such as neonatal sepsis and meningitis. If the newborns carry GBS, about 1%-3% of them will develop early invasive infections, and 5% will lead to death. Neonatal group B streptococcus is associated with perinatal infection and is an important pathogen of severe infectious diseases such as neonatal sepsis and meningitis. This kit accurately diagnoses the infection of group B streptococcus to minimize the incidence rate and harm of it in of pregnant women and newborns as well as the unnecessary economic burden caused by the harm.
Channel
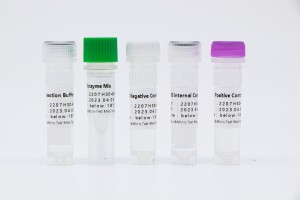
| FAM | GBS nucleic acid |
| ROX | internal reference |
Technical Parameters
| Storage | Liquid: ≤-18℃ |
| Shelf-life | 9 months |
| Specimen Type | Genital tract and rectal secretions |
| Tt | <30 |
| CV | ≤10.0% |
| LoD | 500Copies/mL |
| Specificity | No cross-reactivity with other genital tract and rectal swab samples such as Candida albicans, Trichomonas vaginalis, Chlamydia trachomatis, Ureaplasma urealyticum, Neisseria gonorrhoeae, Mycoplasma hominis, Mycoplasma genitalium, Herpes simplex virus, Human Papillomavirus, Lactobacillus, Gardnerella vaginalis, Staphylococcus aureus, national negative references N1-N10 (Streptococcus pneumoniae, Pyogenic streptococcus, Streptococcus thermophilus, Streptococcus mutans, Streptococcus pyogenes, Lactobacillus acidophilus, Lactobacillus reuteri, Escherichia coli DH5α, and Saccharomyces albicans) and human genomic DNA |
| Applicable Instruments | Applied Biosystems 7500 Real-Time PCR SystemsApplied Biosystems 7500 Fast Real-Time PCR Systems
QuantStudio®5 Real-Time PCR Systems SLAN-96P Real-Time PCR Systems LightCycler®480 Real-Time PCR system LineGene 9600 Plus Real-Time PCR Detection System MA-6000 Real-Time Quantitative Thermal Cycler BioRad CFX96 Real-Time PCR System BioRad CFX Opus 96 Real-Time PCR System |