Short Lead Time for Gastrointestinal - Enterovirus Universal, EV71 and CoxA16 Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test
Short Lead Time for Gastrointestinal - Enterovirus Universal, EV71 and CoxA16 Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test Detail:
Product Name
Enterovirus Universal, EV71 and CoxA16 Nucleic Acid Detection Kit(Fluorescence PCR)
Certificate
CE
Packaging Size
1. Liquid product: 50 tests/kit
2. Freeze-dried product (penicillin vial): 20 tests/kit, 50 tests/kit
3. Freeze-dried product (8-tube strips): 48 tests/kit
Intended Use
This kit is used for in vitro qualitative detection of enterovirus, EV71 and CoxA16 nucleic acids in throat swabs and herpes fluid samples of patients with hand-foot-mouth disease, and provides an auxiliary means for the diagnosis of patients with hand-foot-mouth disease.
Epidemiology
Hand-foot-mouth disease (HFMD) is a common acute infectious disease in children. It mostly occurs in children under 5 years old, and can cause herpes on the hands, feet, mouth and other parts, and a small number of children can cause complications such as myocarditis, pulmonary edema, aseptic meningoencephalitis, etc. Individual children with serious illnesses deteriorate rapidly and are prone to death if not treated promptly.
Currently, 108 serotypes of enteroviruses have been found, which are divided into four groups: A, B, C and D. Enteroviruses that cause HFMD are various, but enterovirus 71 (EV71) and coxsackievirus A16 (CoxA16) are the most common and in addition to HFMD, can cause serious central nervous system complications such as meningitis, encephalitis, and acute flaccid paralysis.
Features
Multiplex PCR Amplification Technology
Internal control: Fully monitor the experimental process to ensure the quality of experiments.
High sensitivity: 500Copies/mL
Wide Coverage Type: Detection of all enteroviruses and phenotyping of EV71 and CoxA16
Channel
FAM: Enterovirus universal RNA
VIC (HEX): CoxA16
ROX: EV71
CY5: Internal control
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 15mins | No |
| 2 | 1 cycle | 95℃ | 10mins | No |
| 3 | 40 cycles | 95℃ | 15s | No |
| 58℃ | 30s | Yes |
Technical Parameters
Storage:
Liquid: ≤-18℃ In dark
Lyophilization: ≤30℃
Shelf-life:
Liquid: 9 months
Lyophilization: 12 months
Specimen Type: Throat swab sample, Herpes fluid
Ct: ≤38
CV: ≤5.0%
LoD: 500Copies/mL
Applicable Instruments: It can match the mainstream fluorescent PCR instruments on the market.
ABI 7500 Real-Time PCR Systems
ABI 7500 Fast Real-Time PCR Systems
SLAN-96P Real-Time PCR Systems
QuantStudio®5 Real-Time PCR Systems
LightCycler®480 Real-Time PCR Systems
LineGene 9600 Plus Real-Time PCR Detection Systems
MA-6000 Real-Time Quantitative Thermal Cycler
BioRad CFX96 Real-Time PCR System
BioRad CFX Opus 96 Real-Time PCR System
Total PCR Solution
● Option 1.

● Option 2.

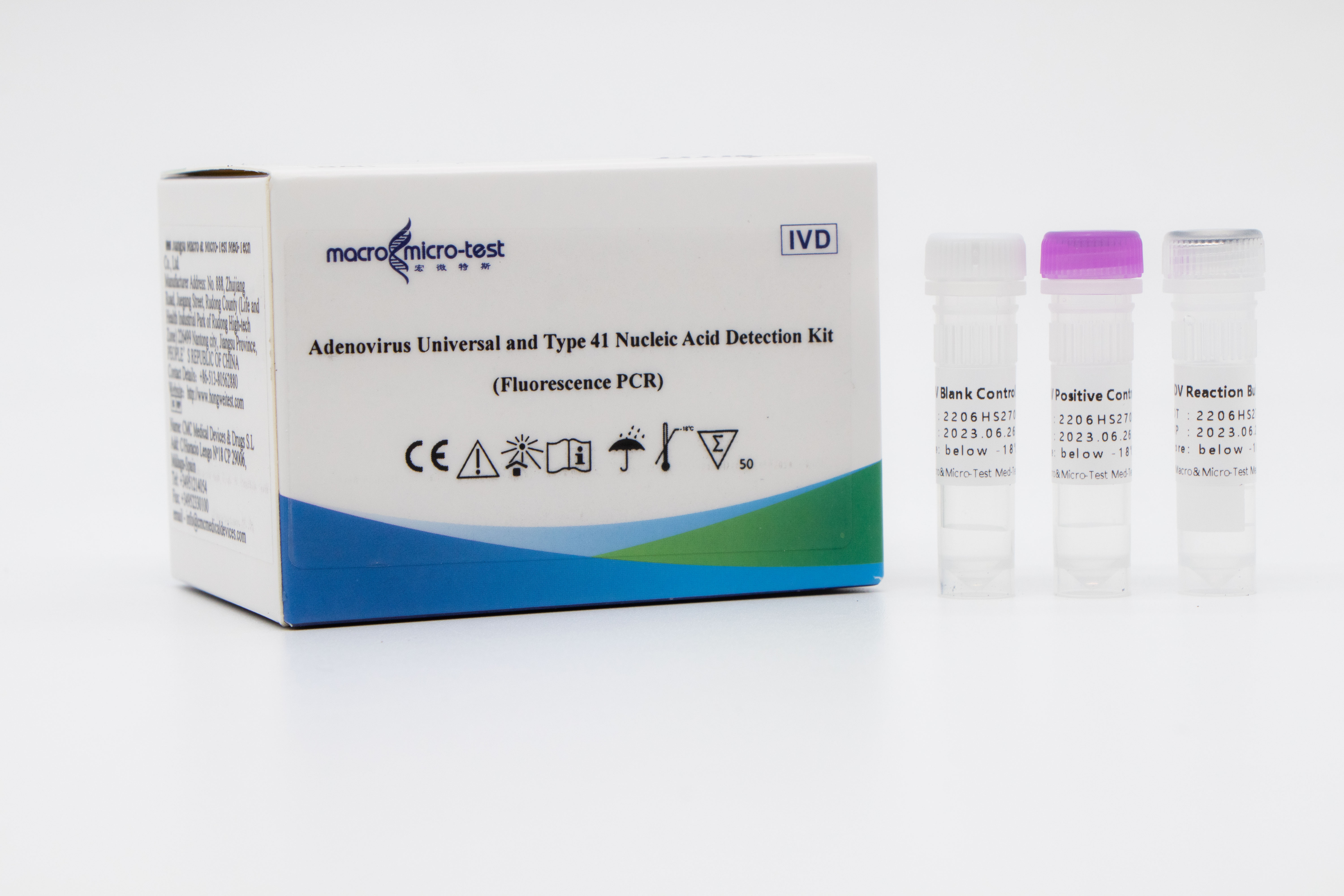
Main Components
Liquid product
| Catalogue Number | Component (50 tests/kit) | Specification | Quantity | Component Description |
| HWTS-EV026A | EV Reaction Buffer | 1.175mL/vial | 1 vial | Enterovirus universal, EV71,CoxA16 and internal control primers, fluorescent probes, reaction buffer, etc |
| EV Enzyme Mix | 75μL/vial | 1 vial | Taq enzyme, reverse transcriptase and UDG enzyme, etc. | |
| EV Positive Control | 1mL | 1 vial | Enterovirus universal, EV71, CoxA16 plasmid and internal control plasmid mixture | |
| EV Blank Control | 1mL | 1 vial | DNase/RNase free H2O |
Lyophilized product
| Component | Character | Catalogue Number | Component Description | ||
| HWTS-EV020A | HWTS-EV020B | HWTS-EV020Z | |||
| Specification/Quantity(20 tests/kit) | Specification/Quantity(50 tests/kit) | Specification/Quantity(48 tests/kit) | |||
| EV Reaction Buffer | lyophilized | 1 bottle | 1 bottle | 8-tube strips, 6 strips | Enterovirus universal, EV71, CoxA16 and internal control primers, fluorescent probes, reaction buffer, Taq enzyme, reverse transcriptase and UDG enzyme, etc. |
| EV Positive Control | lyophilized | 1 vial | 1 vial | 1 vial | Enterovirus universal, EV71, CoxA16 plasmid and internal control plasmid mixture |
| EV Blank Control | Liquid | 1 vial, 600μL/vial | 1 vial, 600μL/vial | 1 vial, 600μL/vial | DNase/RNase free H2O |
| Reconstituted Solution | Liquid | 1 vial, 1mL/vial | 1 vial, 1.45mL/vial | 1 vial, 1.42mL/vial | DNase/RNase free H2O, etc. |
Reference
[1] Xiao Xing-Long et al. Simultaneous detection of human enterovirus 71 and coxsackievirus A16 in clinical specimens by multiplex real-time PCR with an internal amplification control.[J]. Archives of virology, 2009, 154(1) : 121-5.
[2] Cardosa MJ, Perera D, Brown BA et al (2003) Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: comparative analysis of the VP1 and VP4 genes. Emerg Infect Dis 9:461–468.
[3] Oberste MS, Maher K, Flemister MR et al (2000) Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J Clin Microbiol 38:1170–1174.
[4] Tan EL, Chow VT, Quak SH et al (2008) Development of multiplex real-timehybridization probe reverse transcriptase polymerase chain reaction for specific detection and differentiation of Enterovirus 71 and Coxsackievirus A16. Diagn Microbiol Infect Dis (in press).
[5] Singh S, Chow VT, Phoon MC, Chan KP, Poh CL. Direct detection of enterovirus 71 (EV71) in clinical specimens from a hand, foot, and mouth disease outbreak in Singapore by reverse transcription-PCR with universal enterovirus and EV71-specific primers. J Clin Microbiol. 2002 Aug;40(8):2823-7.
Product detail pictures:





Related Product Guide:
To be able to ideal satisfy client's requirements, all of our operations are strictly performed in line with our motto High High-quality, Competitive Price tag, Fast Service for Short Lead Time for Gastrointestinal - Enterovirus Universal, EV71 and CoxA16 Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test , The product will supply to all over the world, such as: Slovakia, Thailand, Iceland, Our products have won an excellent reputation at each of the related nations. Because the establishment of our firm. we've insisted on our production procedure innovation together with the most recent modern day managing method, attracting a sizable quantity of talents within this industry. We regard the solution good quality as our most vital essence character.
This is the first business after our company establish, products and services are very satisfying, we have a good start, we hope to cooperate continuous in the future!