Fetal Fibronectin(FFN) Detection Kit(Immunochromatography) – Macro & Micro-Test
Fetal Fibronectin(FFN) Detection Kit(Immunochromatography) – Macro & Micro-Test Detail:
Product name
Fetal Fibronectin(fFN) Detection Kit(Immunochromatography)
Certificate
CE
Epidemiology
Preterm birth refers to a disease characterized by the interruption of pregnancy after 28 to 37 gestational weeks. Preterm birth is the leading cause of death and disability in most non-hereditary perinatal infants. Symptoms of preterm birth include uterine contractions, changes in vaginal discharge, vaginal bleeding, back pain, abdominal discomfort, a squeezing sensation in the pelvis and cramps.
As an isoform of fibronectin, Fetal Fibronectin (fFN) is a complex glycoprotein with a molecular weight of about 500KD. For pregnant women with signs and symptoms of preterm birth, if fFN ≥ 50 ng/mL between 0 day of 24 weeks and 6 days of 34 weeks, the risk of preterm birth increases within 7 days or 14 days (from the date of specimen testing from cervical vaginal secretions). For pregnant women without signs and symptoms of preterm birth, if fFN is elevated between 0 day of 22 weeks and 6 days of 30 weeks, there will be an increased risk of preterm birth within 6 days of 34 weeks[1].
Features
● Rapid: Read results within 10 minutes
● Easy to use: Only 3 steps
● Convenient: No instrument
● Room temperature: Transportation & storage at 4-30℃ for 24 months
● Accuracy: High sensitivity & specificity
Technical Parameters
| Target region | Fetal Fibronectin |
| Storage temperature | 4℃-30℃ |
| Sample type | Vaginal secretions |
| Shelf life | 24 months |
| Auxiliary instruments | Not required |
| Extra Consumables | Not required |
| Detection time | 10-20 mins |
Work Flow
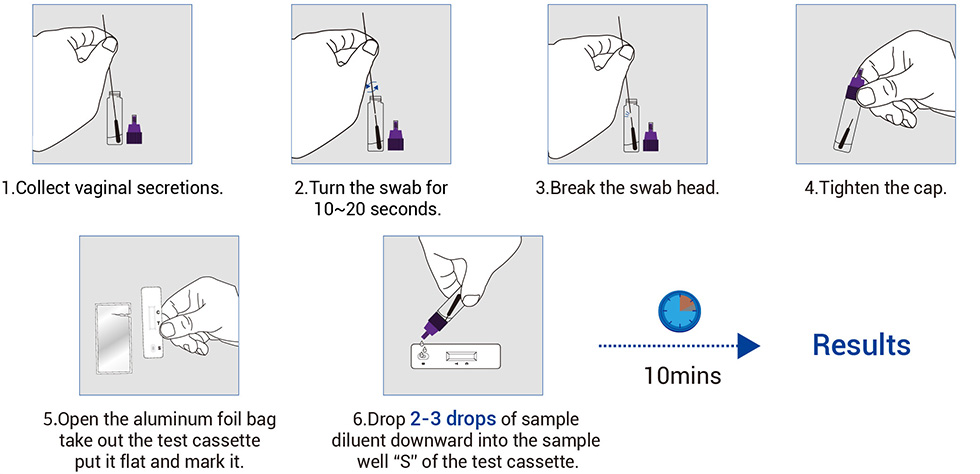
Read the result (10-20 mins)
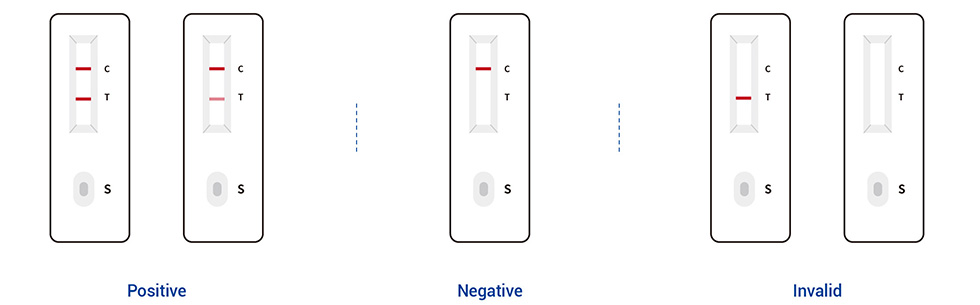
Precautions:
1. Do not read the result after 20 minutes.
2. Please add the sample in strict accordance with the instructions.
3. After opening, please use the product within 1 hour.
4. Women with a history of smoking who have bacterial vaginosis are often tested as positive for fFN. The test results should be combined with other clinical and laboratory data to comprehensively evaluate the possibility of preterm birth.
Main Components
| Catalogue Number | Packaging Size | Components | Specification | Quantity |
| HWTS-PF002A | 5 tests/kit | fFN Test Cassette | 1pc/bag | 5 bags |
| Sample Diluent | 0.3mL/vial | 5 vials | ||
| Cotton Swab | 5pcs/bag | 1 bag | ||
| HWTS-PF002B | 20 tests/kit | fFN Test Cassette | 1pc/bag | 20 bags |
| Sample Diluent | 0.3mL/vial | 20 vials | ||
| Cotton Swab | 20pcs/bag | 1 bag |
Reference
[1] Goldenberg R L, Mercer B M, Iams J D, et al. The preterm prediction study: Patterns of cervicovaginal fetal fibronectin as predictors of spontaneous preterm delivery[J]. American Journal of Obstetrics & Gynecology, 1997, 177( 1):8-12.
Product detail pictures:




Related Product Guide:
Our target is always to satisfy our customers by offering golden support, superior value and high quality for Fetal Fibronectin(FFN) Detection Kit(Immunochromatography) – Macro & Micro-Test , The product will supply to all over the world, such as: Roman, Spain, Spain, We strongly believe that technology and service is our base today and quality will create our reliable walls of future. Only we have better and better quality , could we achieve our customers and ourselves, too. Welcome customers all over the word to contact us for getting further business and reliable relationships. We are always here working for your demands whenever you need.
We feel easy to cooperate with this company, the supplier is very responsible, thanks.There will be more in-depth cooperation.