Detection Kit for Group A Rotavirus and Adenovirus antigens (Colloidal gold) – Macro & Micro-Test
Detection Kit for Group A Rotavirus and Adenovirus antigens (Colloidal gold) – Macro & Micro-Test Detail:
Product name
Detection Kit for Group A Rotavirus and Adenovirus antigens (Colloidal gold)
Certificate
CE
Epidemiology
Rotavirus (Rv) is an important pathogen causing viral diarrhea and enteritis in infants worldwide[1], belonging to the reovirus family, is a double-stranded RNA virus. Group A rotavirus is the main pathogen causing severe diarrhea in infants and young children[2]. Rotavirus with the virus excreted feces, through the fecal route infected patients[3], the proliferation of cells in the duodenal mucosa of the children affected the normal absorption of salts, sugars and water in the intestines of the children, resulting in diarrhea[4].
Adenovirus (Adv) belongs to the Adenovirus family. Type 40 and 41 of Group F can induce diarrhea in infants. They are the second most important pathogen in viral diarrhea in children, next to rotavirus. The main transmission route of adenovirus is fecal-oral transmission, the incubation period of infection is about 10 days, and the main symptoms are diarrhea, accompanied by vomiting and fever[5].
Features
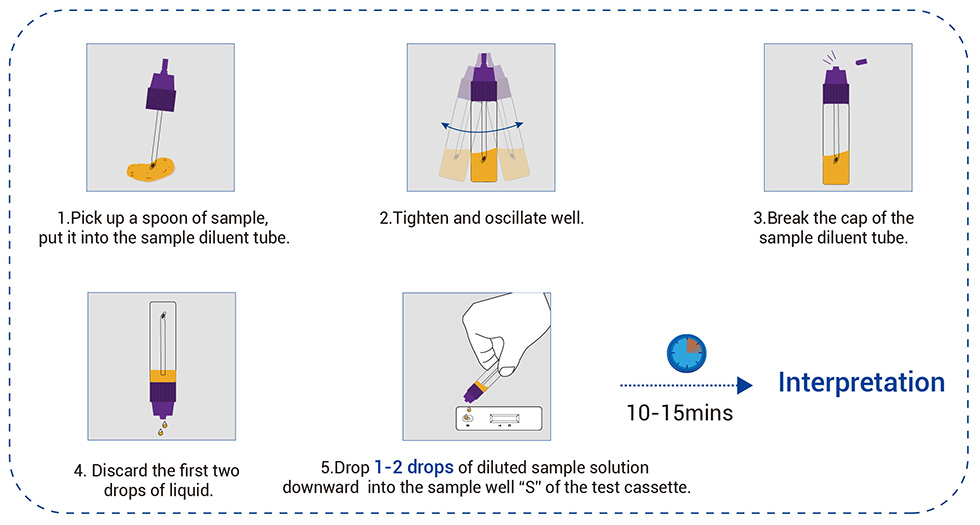
● Rapid: Read results within 10 minutes
● Easy to use: Only 3 steps
● Convenient: No instrument
● Room temperature: Transportation & storage at 2-30℃ for 12 months
● Accuracy: High sensitivity & specificity
Technical Parameters
| Target region | Group A rotavirus and adenovirus |
| Storage temperature | 2℃-30℃ |
| Sample type | Stool samples |
| Shelf life | 12 months |
| Auxiliary instruments | Not required |
| Extra Consumables | Not required |
| Detection time | 10-15 mins |
| Specificity | detection of bacteria by kit include: group B streptococcus, haemophilus influenzae, group C streptococcus, candida albicans, pseudomonas aeruginosa, klebsiella pneumoniae, staphylococcus aureus, enterococcus faecium, enterococcus faecalis, neisseria meningococcus, neisseria gonorrhoeae, acinetobacter , proteus mirabilis, acinetobacter calcium acetate, escherichia coli, proteus vulgaris, gardnerella vaginalis, salmonella, shigella, chlamydia trachomatis, helicobacter pylori, there is no cross reaction |
Work Flow
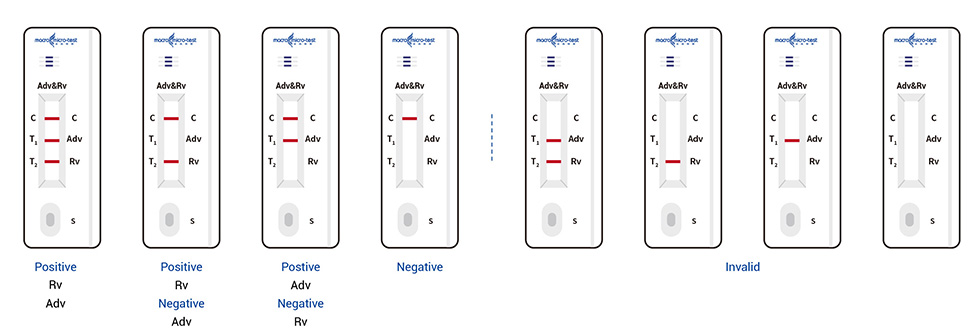
● Read the results (10-15 mins)
Precautions:
1. Do not read the result after 15 mins.
2. After opening, please use the product within half an hour.
3. Please add samples in strict accordance with the instructions.
4. Please perform the test according to the instructions to avoid collecting excessive stool samples or too viscous stool samples, which will cause the diluted sample to block the test cassette.
Main Components
| Catalogue Number | Kit specifications | Composition | Component specifications |
The number of |
| HWTS-EV016A | 50 tests/kit | 1. detection reagent card | 1 piece/package | 50 packages |
| 2. Sample dilution | 1 mL/piece | 50 pieces | ||
| HWTS-EV016B | 10 tests/kit | 1. detection reagent card | 1 piece/ package | 10 packages |
| 2. Sample dilution | 1 mL/piece | 10 pieces |
Reference
[1] Zhang Chunfang, Jia Liying. Epidemiology of rotavirus infection in Asia [J]. Chinese Journal of Contemporary Pediatrics, 2006,89(1): 79-82.
[2] Li Manyuan. Value of rapid detection of rotavirus Group A antigen in clinical diagnosis and treatment of infant diarrhea. Chinese journal of minkang medicine, 2011,23 (14): 1747.
[3] Xu Xiaoling. Analysis of rotavirus antigen in feces of children with diarrhea and its clinical significance. Chinese journal of medical research, 2006,6 (12): 1404-1405.
[4] Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis[J]. N Engel J Med, 2006,354(1): 11-22.
[5] SCHMITZ, H. et al. Worldwide epidemiology in human Rotavirus infections, J. Epidemiology, 117:455-466.
Product detail pictures:






Related Product Guide:
To consistently enhance the management method by virtue of the rule of sincerely, fantastic religion and top quality are the base of business development, we extensively absorb the essence of associated goods internationally, and constantly acquire new merchandise to satisfy the needs of shoppers for Detection Kit for Group A Rotavirus and Adenovirus antigens (Colloidal gold) – Macro & Micro-Test , The product will supply to all over the world, such as: Rwanda, Guatemala, Namibia, We believe with our consistently excellent service you can get the best performance and cost least products from us for a long term . We commit to provide better services and create more value to all our customers. Hope we can create a better future together.
The quality of the products is very good, especially in the details, can be seen that the company work actively to satisfy customer's interest, a nice supplier.