As monkeypox continues to pose a global health challenge, having a diagnostic tool that is both trustworthy and efficient has never been more critical. Jiangsu Macro & Micro-Test Med-Tech is proud to announce that our Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR) has been selected for the World Health Organization Emergency Use Listing (WHO EUL)—a recognition that underscores its compliance with international standards of quality, safety, and performance.
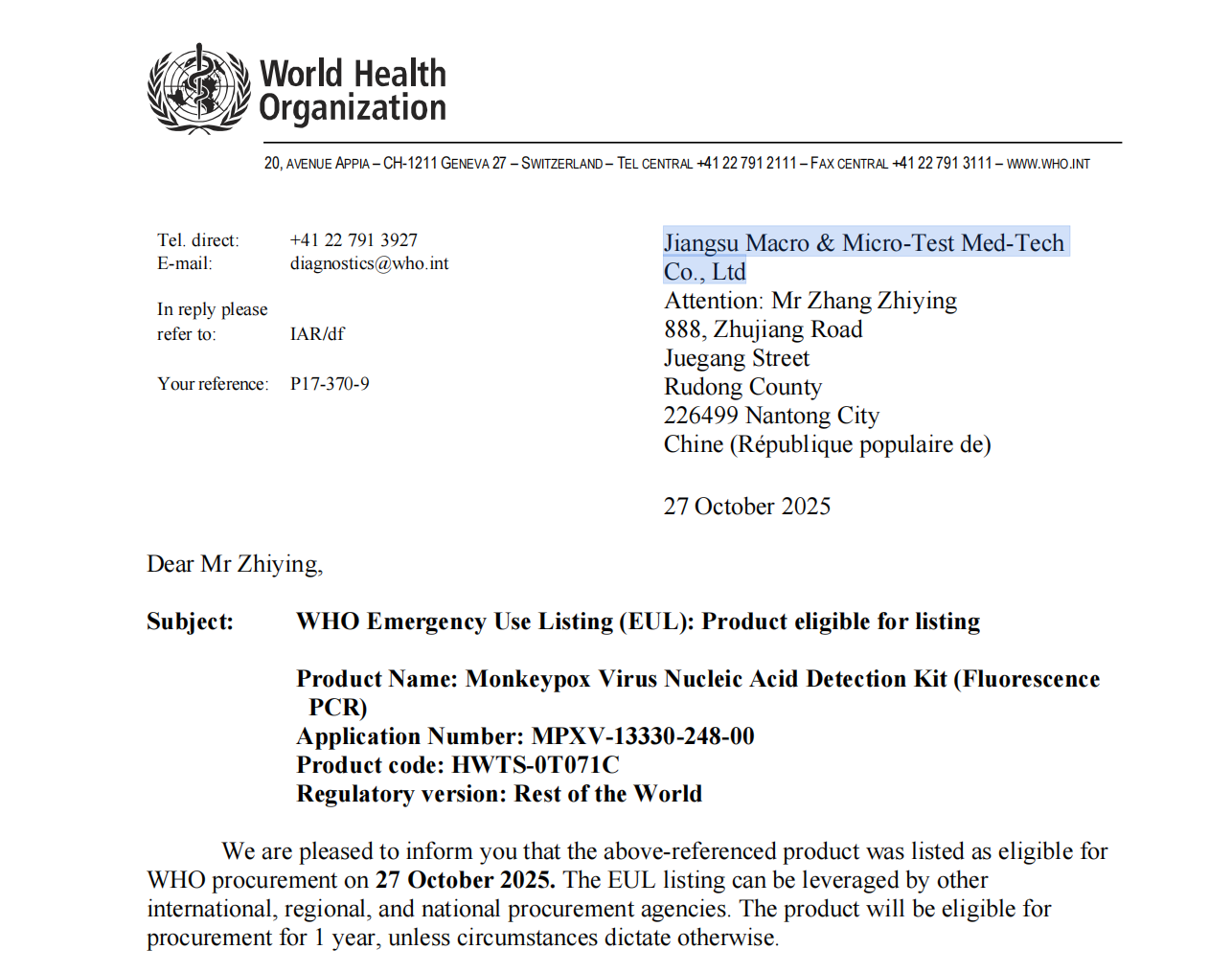
This isn’t just another approval—it’s your assurance of a ready-to-deploy, precision-driven solution designed for labs, hospitals, and public health programs worldwide.
Why Choose Our Monkeypox Detection Kit?
✅ Flexible Sampling
Rash fluid, throat swab or serum sample;
✅Dual-Target Gene Design
Ensures highly accurate detection with a sensitivity of 200 copies/mL, enabling early identification even in low viral load scenarios.
✅ Broad Coverage of Clades I & II
Comprehensive detection of known monkeypox virus strains, backed by outstanding clinical performance:
-PPA: 100%
-NPA: 99.40%
-OPA: 99.64%
-Kappa: 0.9923
✅ Exceptional Specificity
No cross-reactivity with smallpox virus, vaccinia virus, cowpox virus, mousepox virus, herpes simplex virus, varicella-zoster virus, or human genome—so you can trust every result.
✅ Fast & Streamlined Workflow
From sample to result within 40 minutes, with flexible sampling options including rash fluid, throat swab, and serum.
✅ Built-In Quality Control
An internal control monitors the entire process, ensuring reliability at every step.
✅ Platform Flexibility
Compatible with mainstream fluorescence PCR systems, making it easy to integrate into your existing lab setup.
✅ Multi-Scenario Use
Ideal for hospitals, CDC labs, and research institutes—wherever accurate and rapid diagnosis is needed.
A Tool for Global Health Readiness
Since 2022, monkeypox has spread across 140+ countries, with over 160,000 confirmed cases reported globally. While case numbers have recently declined, the risk of viral mutation and gaps in regional surveillance remain.
Our WHO EUL-listed kit provides a dependable tool to help:
-Strengthen early warning systems
-Support rapid case isolation and containment
-Facilitate cross-border collaboration and research
Designed for Impact, Built for Scale
As an innovative IVD manufacturer based in Jiangsu, China, Macro & Micro-Test is committed to developing diagnostic solutions that meet real-world needs. This WHO EUL endorsement enables faster international procurement and distribution—especially in resource-limited settings—helping ensure equitable access to reliable testing.
Ready to Enhance Your Diagnostic Capability?
Contact us to learn more about our Monkeypox Detection Kit or explorecollaborative opportunities.
Email: marketing@mmtest.com
Website: www.mmtest.com
—Precision Diagnosis Shapes a Better Life—
Post time: Oct-30-2025
