Group B Streptococcus (GBS) is a common bacterium but poses a significant, often silent, threat to newborns. While typically harmless in healthy adults, GBS can have devastating consequences if passed from mother to baby during childbirth. Understanding carrier rates, the potential impact, and the critical importance of timely and accurate testing is key to safeguarding infant health.
The Silent Prevalence of GBS
Group B Strep is remarkably common. Studies indicate that approximately 1 in 4 pregnant individuals carry GBS bacteria in their rectum or vagina, usually without any symptoms. This makes routine screening the only reliable way to identify carriers and prevent transmission.
The Grave Risk to Newborns
When transmitted to a newborn, GBS can cause serious, life-threatening infections within the first week of life (early-onset disease) or later (late-onset disease). These infections include:
Sepsis (bloodstream infection): A leading cause of newborn mortality.
Pneumonia: Infection in the lungs.
Meningitis: Infection of the fluid and lining surrounding the brain and spinal cord, potentially leading to long-term neurological damage.
Early-onset GBS disease remains a significant cause of newborn illness and death globally. Prompt intervention is crucial for survival and minimizing long-term complications.
The Lifesaving Power of Screening & Prophylaxis
The cornerstone of prevention is universal GBS screening (recommended between 36-37 weeks gestation by organizations like ACOG) and administering intrapartum antibiotic prophylaxis (IAP) to identified carriers during labor. This simple intervention drastically reduces the risk of transmission and early-onset disease.
The Challenge: Timeliness and Accuracy in Testing
Traditional GBS screening methods face hurdles that can impact care, especially in urgent scenarios like preterm labor or premature rupture of membranes (PROM):
Time Delays: Standard culture methods take 18-36 hours for results – time often unavailable when labor progresses quickly.
False Negatives: Culture sensitivity can drop significantly (studies suggest around 18.5% false negatives), partly due to recent antibiotic use masking growth.
Limited Point-of-Care Options: While faster immunoassays exist, they often lack sufficient sensitivity. Molecular tests offer accuracy but traditionally required specialized labs and took hours.
The Critical Need: Rapid, Reliable Results at the Point of Care
The limitations of traditional testing underscore the immense value of rapid, accurate, point-of-care GBS diagnostics. Timely detection during labor is essential for:
Effective Decision-Making: Ensuring IAP is administered promptly to all carriers.
Optimizing Newborn Care: Allowing for appropriate monitoring and early treatment if needed.
Reducing Unnecessary Antibiotics: Avoiding broad antibiotic use in individuals with confirmed negative status.
Managing Urgent Situations: Providing crucial information rapidly during preterm labor or PROM.
Advancing Care: The Promise of Rapid Molecular GBS Testing
Innovative solutions like the Macro & Micro-Test GBS+Easy Amp System are transforming GBS detection:
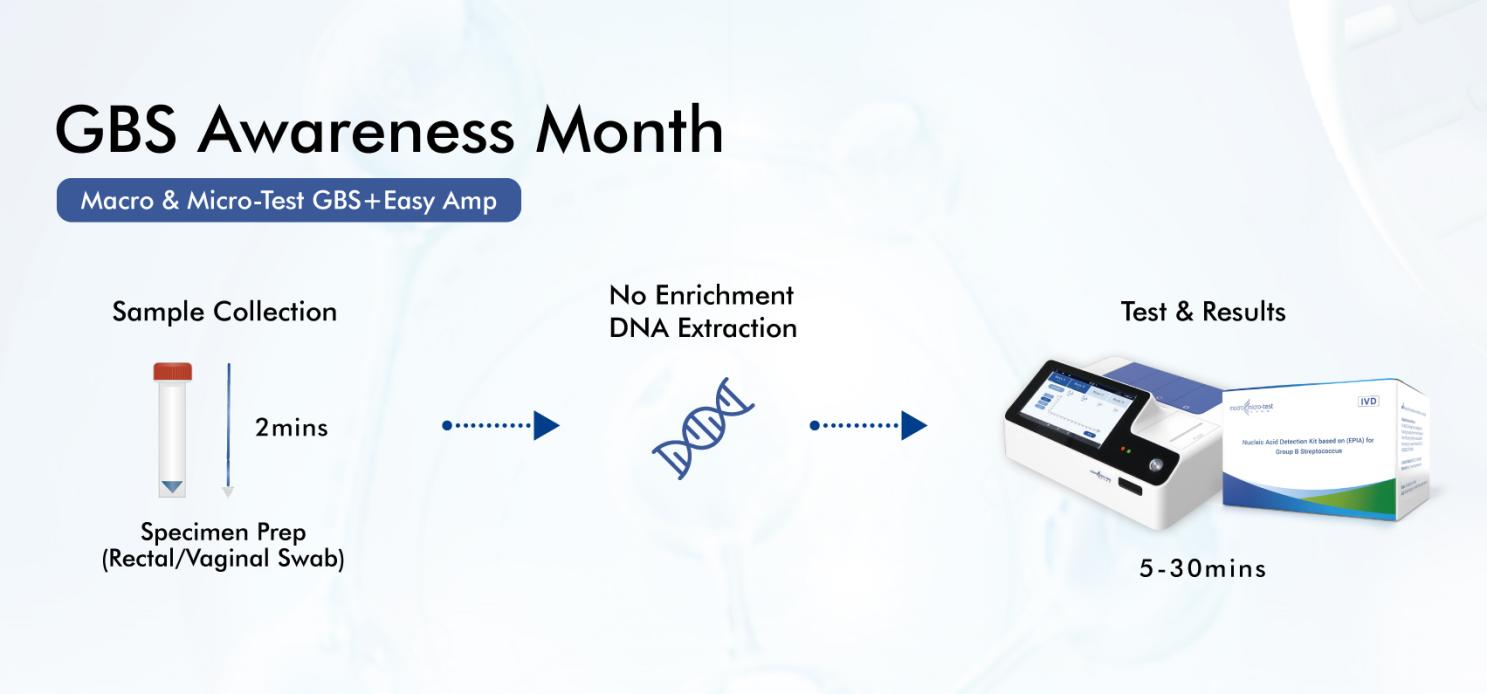
Unprecedented Speed: Delivers positive results in just 5 minutes, enabling immediate clinical action.
High Accuracy: Molecular technology provides reliable results, minimizing dangerous false negatives.
True Point-of-Care: The Easy Amp System facilitates on-demand testing directly in labor & delivery or antenatal clinics using standard vaginal/rectal swabs.
Operational Flexibility: Independent system modules allow testing to adapt to clinical workflow needs.

Prioritizing universal screening and leveraging rapid, reliable diagnostics is the key pathway to these goals. It ensures timely interventions when they matter most, directly reducing the burden of early-onset GBS disease.
Contact us at marketing@mmtest.com for detailed product information and distribution policies.
Post time: Dec-26-2025
