Since May 2022, mpox cases have been reported in many non-endemic countries in the world with community transmissions.
On 26 August, the World Health Organization (WHO) launched a global Strategic Preparedness and Response Plan to stop outbreaks of human-to-human transmission of mpox through coordinated global, regional, and national efforts. This follows the declaration of a public health emergency of international concern by the WHO Director-General on 14 August.
It should be noted that mpox outbreak this time is different from that in 2022, which was mainly spreading among men who have sex with men, and the mortality rate of infected people was less than 1%.
The recent prevalent strain “Clade Ib”, which is a variant of Clade I, has a higher mortality rate. This new variant began to spread in DRC last September, initially among sex workers, and has now spread to other groups, with children being particularly susceptible.
The Africa CDC said in a report last month that mpox outbreaks have been found in 10 African countries this year, including DRC, which has reported 96.3% of all cases in Africa this year and 97% of the deaths. It is worth noting that nearly 70% of the cases in DRC are children under the 15s, and this group accounts for 85% of the deaths in the country.
Mpox is a zoonosis caused by the mpox virus with the incubation period of 5 to 21 days, mostly 6 to 13 days. The infected person will have symptoms such as fever, headache and swollen lymph nodes, followed by rash on the face and other parts of the body, which gradually develops into pustules and lasts for about a week before scabbing. The case is contagious from the onset of symptoms until the scabs fall off naturally.
Macro & Micro-Test is providing rapid tests, molecular kits and sequencing solutions for mpox virus detection, assisting in-time mpox virus diagnosis, supervision of its origin, lineage, transmission and genomic variations:
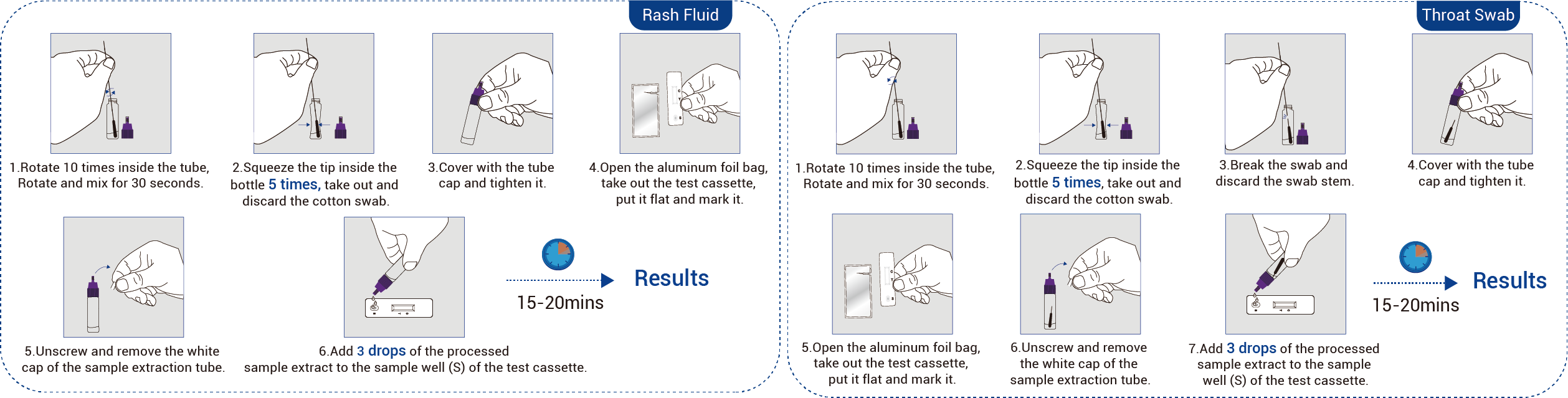
Monkeypox Virus Antigen Detection Kit (Immunochromatography)
Easy sampling (rash fluid/throat sample) and fast result within 10-15 min;
High sensitivity with LoD of 20pg/mL covering Clade I & II;
High specificity with no cross-reactivity with smallpox virus, varicella zoster virus, rubella virus,herpes simplex virus, etc.
OPA of 96.4% compared with NAATs;
Wide application such as customs, CDCs, pharmacies, clinics, hospitals or at home.
Monkeypox-virus IgM/IgG Antibody Detection Kit (Immunochromatography)
Easy instrument-free operation and fast result within 10 min;
High sensitivity and specificity covering Clade I & II;
Identifies IgM and IgG to decide mpox infection stages;
Wide application such as customs, CDCs, pharmacies, clinics, hospitals or at home;
Appropriate for large-scale screening of suspected mpox infection.
Monkeypox Virus Nucleic Acid Detection Kit (Enzymatic Probe Isothermal Amplification)
High sensitivity with LoD of 200 Copies/mL with IC, equals to florescence PCR;
Easy operation: Lysed sample added to the lyophilized reagent tube for direct on-demand amplification enabled by independent modules of Easy Amp System;
High specificity without cross reactivity with smallpox virus, vaccinia virus, cowpox virus, mousepox virus, herpes simplex virus, varicella-zoster virus, and human genome, etc.;
Easy sampling (rash fluid/oropharyngeal swab) and fastest positive result within 5 min;
Excellent clinical performance covering Clade I & II with PPA of 100%, NPA of 100%, OPA of 100% and Kappa Value of 1.000 compared with Fluorescence PCR kit;
Lyophilized version requiring only room temperature transport and storage enables accessibility in all regions;
Flexible scenarios in clinics, healthcare center, together with Easy Amp for on-demand detection;
Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR)
Dual gene targeted with high sensitivity with LoD of 200 copies/mL ;
Flexible sampling of rash fluid, throat swab and serum;
High specificity without cross reactivity with smallpox virus, vaccinia virus, cowpox virus, mousepox virus, herpes simplex virus, varicella-zoster virus, and human genome, etc.;
Easy operation: rapid sample lysis by sample release reagent to be added to the reaction tube;
Rapid detection: result within 40 min;
Accuracy ensured by internal control supervising the whole detection process;
Excellent clinical performance covering Clade I & II with PPA of 100%, NPA of 99.40%, OPA of 99.64% and Kappa Value of 0.9923 compared with sequencing;
Lyophilized version requiring only room temperature transport and storage enables accessibility in all regions;
Compatible with mainstream Fluorescence PCR Systems;
Flexible scenarios for hospitals, CDCs and labs;
Orthopox Virus Universal Type/Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR)
Full coverage: tests all the 4 orthopox viruses that can infect human and the prevalent mpox (Clade I&II included) in the single test to avoid missed detection;
High sensitivity with LoD of 200 copies/mL;
High specificity without cross reactivity with other pathogens causing rashes such as herpes simplex virus, varicella-zoster virus, and human genome, etc.;
Easy operation: rapid sample lysis by sample release reagent to be added to the single tube reaction buffer;
Rapid detection: rapid amplification with result within 40 min;
Accuracy ensured by internal control supervising the whole detection process;
Compatible with mainstream Fluorescence PCR Systems;
Flexible scenarios for hospitals, CDCs and labs;
Monkeypox Virus Typing Nucleic Acid Detection Kit (Fluorescence PCR)
Simultaneously identifies the Clade I and Clade II, that is significant for understanding the epidemiological characteristics of the virus, tracing its transmission, and formulating targeted prevention and control measures.
High sensitivity with LoD of 200 copies/mL ;
Flexible sampling of rash fluid, oropharyngeal swab and serum;
High specificity without cross reactivity between Clade I and II, other pathogens causing rashes such as herpes simplex virus, varicella-zoster virus, and human genome, etc.;
Easy operation: rapid sample lysis by sample release reagent to be added to the single tube reaction buffer;
Rapid detection: result within 40 min;
Accuracy ensured by internal control supervising the whole detection process;
Lyophilized version requiring only room temperature transport and storage enables accessibility in all regions;
Compatible with mainstream Fluorescence PCR Systems;
Flexible scenarios for hospitals, CDCs and labs;
Monkey Virus Universal Whole Genome DetectionKit (Multi-PCR NGS)
Newly developed Monkeypox Virus Whole Genome Detection Kit by Macro & Micro-Test for different scenarios, combined with the ONT nanopore sequencer, can acquire MPXV whole genome sequence with a coverage of not less than 98% within 8 hours.
Easy to operate: patented one-step amplification technology, the whole genome sequence of mpox virus can be obtained by one-round amplification;
Sensitive and accurate: detects samples low to 32CT, and 600bp amplicon nanopore sequencing can meet higher quality genome assembly;
Ultra-fast: ONT can complete genome assembly within 6-8 hours;
Wide compatibility: with ONT, Qi Carbon, SALUS Pro, lllumina, MGI and other mainstream 2nd and 3rd generation sequencers.
Ultra-sensitive Monkey Virus Whole Genome Detection Kit-Illumina/MGI (Multi-PCR NGS)
Regarding large numbers of the existing 2nd generation sequencers worldwide, Macro & Micro-Test has also developed ultra-sensitive kits adapting to the mainstream sequencers to achieve low-concentration sample viral genome sequencing;
Efficient amplification: 1448 pairs of 200bp amplicon ultra-dense primer design for high amplification efficiency and uniform coverage;
Easy operation: Mpox virus llumina/MGI library can be obtained through two-round amplification in 4 hours, avoiding complex library construction steps and reagent costs;
High sensitivity: detects samples low to 35CT, effectively avoiding false negative results caused by fragment degradation or low copy number;
Wide compatibility with mainstream 2nd generation sequencers such as lllumina, Salus Pro or MGI; Up to now, more than 400 clinical cases have been completed.
Post time: Aug-28-2024