The global spread of highly pathogenic H5 avian influenza continues to intensify. Across Europe, outbreaks have surged, with Germany alone culling nearly one million birds. In the United States, two million egg-laying hens have been destroyed due to infection, and H5N1 has now been detected in dairy herds across multiple states. Cambodia has also reported several human H5N1 cases, including six death.
The growing risk of cross-species transmission highlights an urgent threat to human health. Whether for clinical screening in hospitals, epidemic surveillance by public health authorities, or health checks at border control, rapid and accurate influenza testing is the critical “first line of defense” in global prevention and control efforts.
Macro & Micro-Test — Advanced Molecular Detection for Influenza Control
Macro & Micro-Test offers a comprehensive portfolio of fluorescent PCR detection kits for multiple influenza virus subtypes — including H1N1, H3, H5, H7, H9, and H10.
These high-performance kits enable early detection and accurate subtype identification, helping medical institutions and laboratories take timely preventive action.
Versatile Applications to Meet Diverse Testing Needs
Subtype-Specific Detection — Targeting High-Risk Strains
-H5 Subtype Detection Kit: Detects highly pathogenic H5 strains such as H5N1 that can infect humans. Ideal for rapid screening of suspected cases in medical facilities.
-H9 Subtype Detection Kit: Targets low-pathogenic H9 viruses occasionally found in humans. Suitable for health monitoring of high-risk populations (e.g., poultry workers, travelers), helping prevent silent transmission.
-H3/H10 Subtype Detection Kit: Designed to detect both common seasonal subtypes (H3) and rare sporadic strains (H10), filling critical gaps in influenza detection.
Multiplex Detection — Comprehensive Screening in a Single Test
-H5/H7/H9 Triple Detection Kit: Detects three major high-risk subtypes in one reaction. Perfect for large-scale screening during peak flu seasons or in densely populated areas.
-Six-Plex Detection Kit: Simultaneously identifies H1N1, H3, H5, H7, H9, and H10 — the ideal choice for hospitals and CDC laboratories handling complex samples (e.g., patients with unexplained fevers), minimizing the chance of missed infections.
Proven Performance — Accurate, Sensitive, and Reliable
-High Accuracy: PCR-fluorescent probe technology targets conserved viral gene regions. Positive/negative reference compliance rate reaches 100%, ensuring dependable results without false positives or negatives.
-High Sensitivity: Detects as low as 500 copies/mL, allowing early identification of low viral loads and providing valuable time for intervention.
-Broad Compatibility: Works seamlessly with mainstream real-time PCR systems (ABI, Hongshi, Bio-Rad, etc.) — plug and play with no additional equipment required.
-Stringent Quality Control: Built-in internal controls monitor the entire testing process to ensure result authenticity and reliability.
Who Should Use It?
Medical Institutions (Fever Clinics, Infectious Disease Departments): For accurate screening and diagnosis of influenza-like illnesses, especially potential H5N1 cases.
Centers for Disease Control (CDC): For influenza surveillance, outbreak tracing, and monitoring close contacts — providing essential data for public health decision-making.
Customs and Border Quarantine: For rapid screening of inbound travelers’ swab samples to prevent cross-border virus transmission.
Community Health Centers: For batch screening during flu outbreaks and health monitoring of frontline or high-risk personnel (e.g., healthcare workers, port staff).
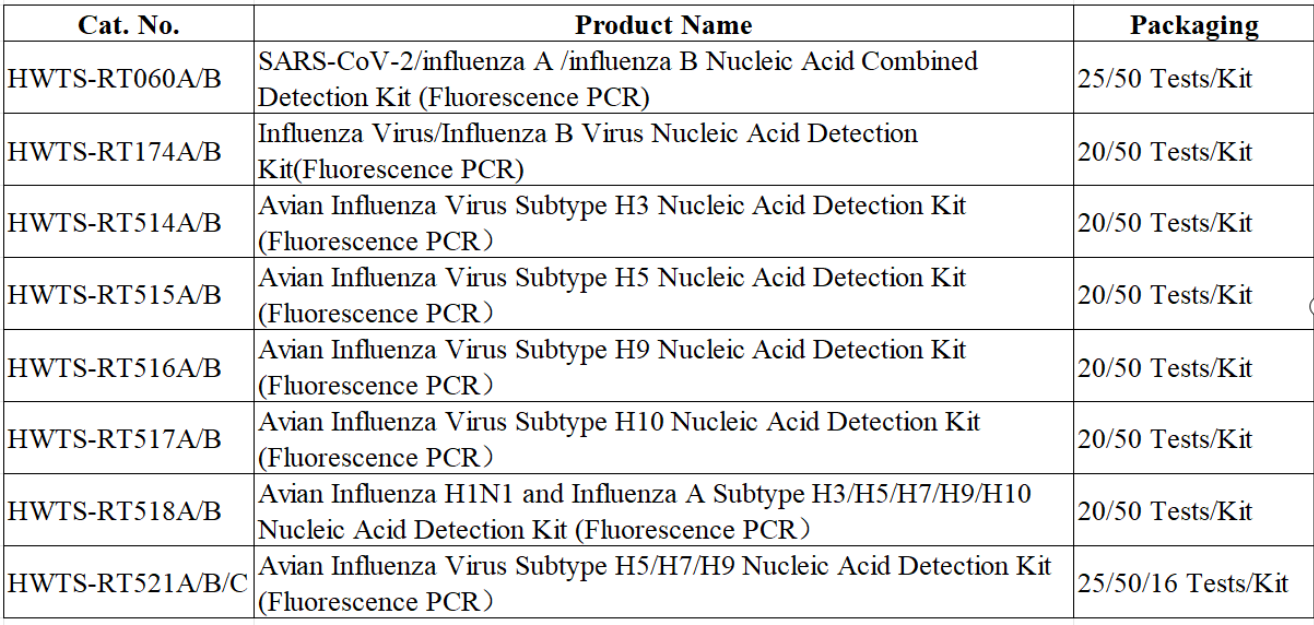
Product Information
Macro & Micro-Test — Precision Testing for a Safer Future.
Empowering global efforts in early detection, rapid response, and effective influenza control.
Post time: Nov-13-2025
