Point mutations in the KRAS gene are implicated in a range of human tumors, with mutation rates of approximately 17%–25% across tumor types, 15%–30% in lung cancer, and 20%–50% in colorectal cancer. These mutations drive treatment resistance and tumor progression through a key mechanism: the P21 protein encoded by KRAS functions downstream of the EGFR signaling pathway. Once KRAS is mutated, it perpetually activates downstream signaling, rendering upstream EGFR-targeted therapies ineffective and leading to sustained malignant cell proliferation. As a result, KRAS mutations are associated with resistance to EGFR tyrosine kinase inhibitors in lung cancer and to anti-EGFR antibody therapies in colorectal cancer.
In 2008, the National Comprehensive Cancer Network (NCCN) established clinical guidelines recommending KRAS mutation testing for all patients with metastatic colorectal cancer (mCRC) prior to treatment. The guidelines highlight that the majority of activating KRAS mutations occur in codons 12 and 13 of exon 2. Thus, rapid and accurate KRAS mutation detection is essential for guiding appropriate clinical therapy.
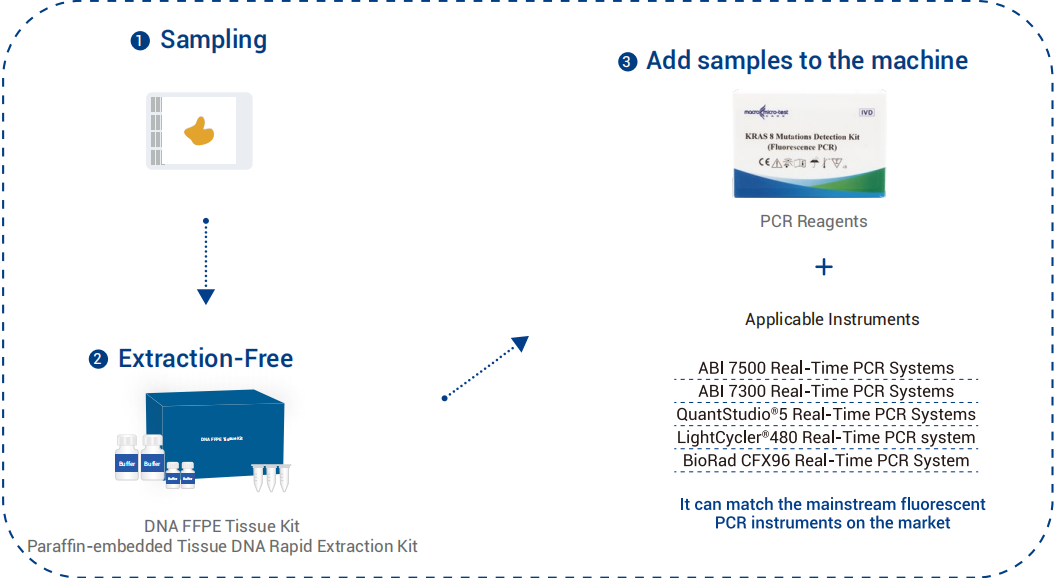
Why KRAS Testing Is Critical in Metastatic Colorectal Cancer (mCRC)
Colorectal cancer (CRC) is not a single disease but a collection of molecularly distinct subtypes. KRAS mutations—present in approximately 40–45% of CRC patients—act as a constant “on” switch, promoting cancer growth independent of external signals. For patients with mCRC, KRAS status determines the efficacy of anti-EGFR monoclonal antibodies such as Cetuximab and Panitumumab:
Wild-Type KRAS: Patients are likely to benefit from anti-EGFR treatment.
Mutant KRAS: Patients gain no benefit from these agents, risking unnecessary side effects, increased costs, and delays in effective therapy.
Accurate and sensitive KRAS testing is therefore the cornerstone of personalized treatment planning.
The Detection Challenge: Isolating the Mutation Signal
Traditional methods often lack sensitivity for low-abundance mutations, particularly in samples with low tumor content or after decalcification. The difficulty lies in distinguishing the faint mutant DNA signal against a high wild-type background—much like finding a needle in a haystack. Inaccurate results can lead to misinformed treatment and compromised outcomes.
Our Solution: Precision-Engineered for Confident Mutation Detection
Our KRAS Mutation Detection Kit integrates advanced technologies to overcome these limitations, delivering exceptional accuracy and reliability for mCRC therapy guidance.
How Our Technology Ensures Superior Performance
- Enhanced ARMS technology (Amplification Refractory Mutation System): Builds upon ARMS technology, incorporating proprietary enhancer technology to increase detection specificity.
- Enzymatic Enrichment: Utilizes restriction endonucleases to digest the majority of the human genome’s wild-type background, sparing mutant types, thus enhancing detection resolution and reducing non-specific amplification due to high genomic background.
- Temperature Blocking: Introduces specific temperature steps in the PCR process, causing mismatch between mutant primers and wild-type templates, thereby reducing wild-type background and improving detection resolution.
- High Sensitivity: Accurately detects as low as 1% mutant DNA.
- Excellent Accuracy: Uses internal standards and UNG enzyme to prevent false positive and negative results.
- Simple and Rapid: Completes testing in approximately 120 minutes, utilizing two reaction tubes to facilitate the detection of eight distinct mutations, yielding objective and reliable results.
- Instrument Compatibility: Adapts to various PCR instruments.
Precision medicine in colorectal cancer starts with precise molecular diagnostics. By adopting our KRAS Mutation Detection Kit, your laboratory can deliver definitive, actionable results that directly shape a patient’s treatment pathway.
Empower your lab with reliable, cutting-edge technology—and enable truly personalized care.
Contact us: marketing@mmtest. Com
Learn more about integrating this advanced solution into your diagnostic workflow.
#Colorectal #Cancer #DNA #Mutation #Precision #Targeted #Treatment #Cancer
Unlocking Precision Medicine in Colorectal Cancer
https://www.linkedin.com/posts/macro-micro-ivd_colorectal-cancer-dna-activity-7378358145812930560-X4MN?utm_source=share&utm_medium=member_desktop&rcm=ACoAADjGw3MB2hg53ctNLAYoEtkigA_pq_iOpoM
Post time: Sep-30-2025