Lung cancer remains a global health challenge, ranking as the second most commonly diagnosed cancer. In 2020 alone, there were over 2.2 million new cases worldwide. Non-small cell lung cancer (NSCLC) represents more than 80% of all lung cancer diagnoses, highlighting the urgent need for targeted and effective treatment strategies.
EGFR mutations have emerged as a cornerstone in the personalized treatment of NSCLC. EGFR tyrosine kinase inhibitors (TKIs) offer a revolutionary approach by blocking cancer-driving signals, inhibiting tumor growth, and promoting cancer cell death—all while minimizing damage to healthy cells.
Leading clinical guidelines, including the NCCN, now mandate EGFR mutation testing before initiating TKI therapy, ensuring the right patients receive the right drugs from the very beginning.
EGFR mutations have emerged as a cornerstone in the personalized treatment of NSCLC. EGFR tyrosine kinase inhibitors (TKIs) offer a revolutionary approach by blocking cancer-driving signals, inhibiting tumor growth, and promoting cancer cell death—all while minimizing damage to healthy cells.
Leading clinical guidelines, including the NCCN, now mandate EGFR mutation testing before initiating TKI therapy, ensuring the right patients receive the right drugs from the very beginning.
Introducing the Human EGFR Gene 29 Mutations Detection Kit (Fluorescence PCR)
Precision Detection for Confident Treatment Decisions
Macro & Micro-Test’s EGFR detection kit enables rapid and accurate identification of 29 key mutations across exons 18–21 in both tissue and liquid biopsies— empowering clinicians to tailor therapy with confidence.
Why Choose Macro & Micro-Test’s EGFR Testing Kit?
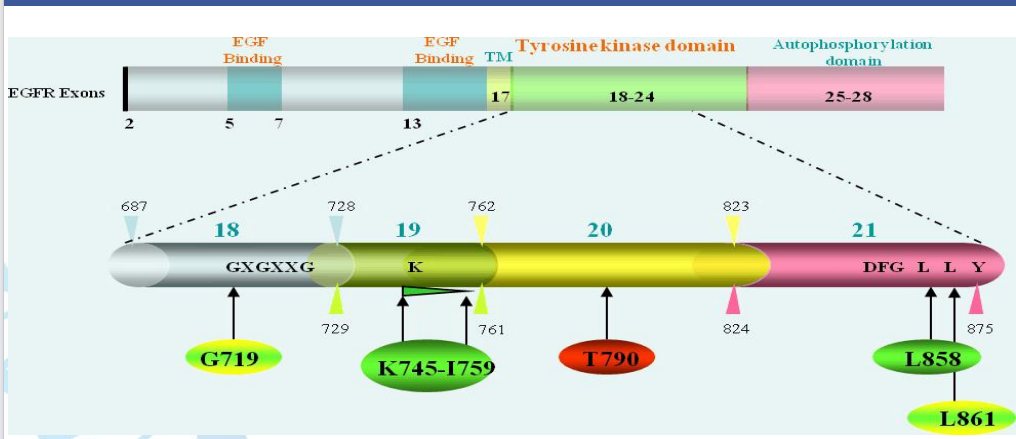
The kit detects 29 common EGFR gene mutations in exons 18-21 from tissue or blood samples of NSCLC patients, covering drug sensitivity and resistance sites to guide the use of targeted drugs like gefitinib and osimertinib.
- 1.Improved ARMS Technology: Enhanced ARMS with patented enhancer for higher specificity;
- 2.Enzymatic Enrichment: Reduces the wild-type background by enzymatic digestion, improving detection accuracy and reducing non-specific amplification due to high genomic background;
- 3.Temperature Blocking: Adds specific temperature phases in the PCR process, reducing mismatches and increasing detection accuracy;
- 4.High Sensitivity: Detects mutations as low as 1% mutation ;
- 5.Great Accuracy: Internal control and UNG enzyme to minimize false results;
- 6.Efficiency: Objective results within 120 min
- 7.Dual Sample Support – Optimized for both tissue and blood samples, offering flexibility in clinical practice
- 8.Wide compatibility: Widely compatible with mainstream PCR instruments on the market;
- 9.Shelf-life: 12 months.
Guide Therapy with Confidence
The kit helps to maximize clinical outcomes and stay ahead of resistance with critical sensitivity and resistance mutations.
Expand Your Precision Oncology Portfolio
Explore our full range of mutation detection solutions for KRAS, BRAF, ROS1, ALK, BCR-ABL, TEL-AML1, and more—all designed to support comprehensive biomarker-driven care.
Learn more: https://www.mmtest.com/oncology/
Contact our team: marketing@mmtest.com
Post time: Sep-23-2025