Macro & Micro-Test is pleased to announce that its independently developed Fully Automated All-in-One Cartridge-based Library Preparation System (HWTS-AIOS) has officially received medical device registration approval from China’s National Medical Products Administration (NMPA).
This milestone confirms that AIOS meets stringent national requirements for safety, performance, compliance, and clinical reliability, marking a significant step forward in bringing standardized, automated next-generation sequencing (NGS) library preparation into routine clinical and public health applications.
True “Sample-to-Library” Automation in a Closed System
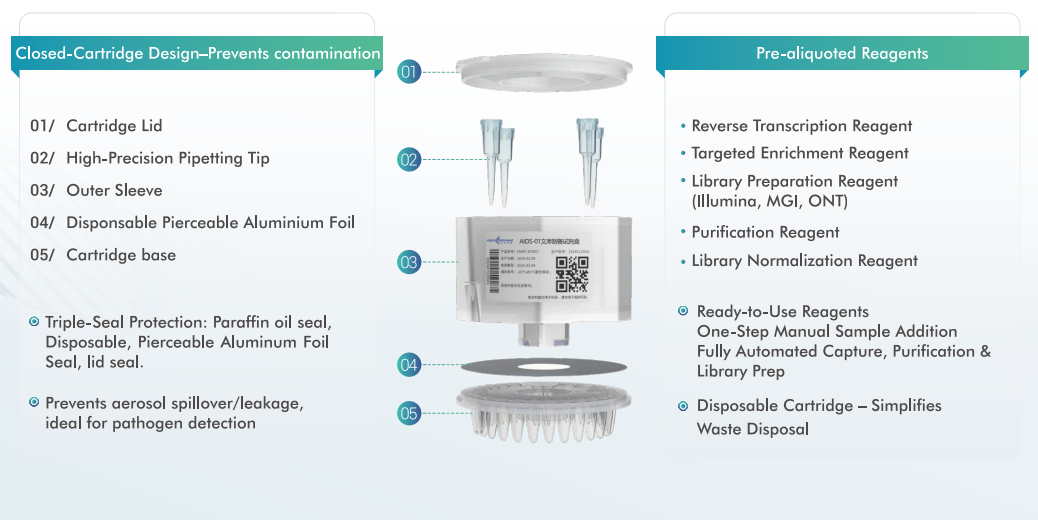
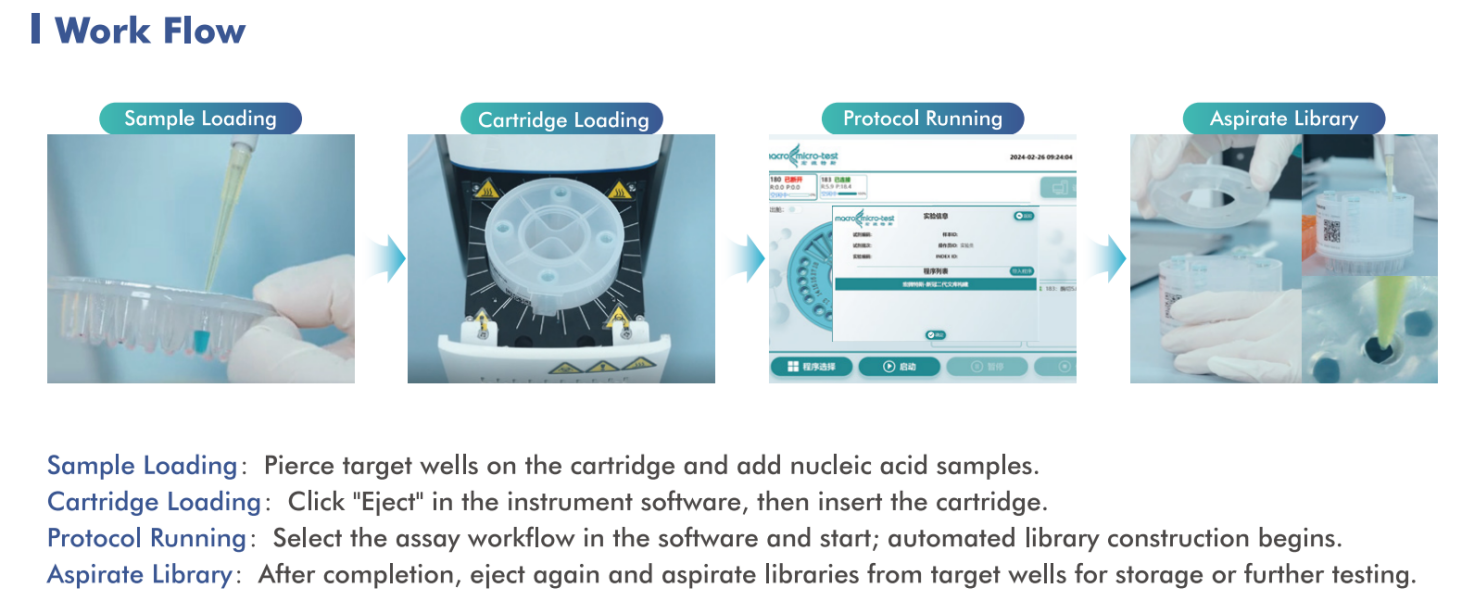
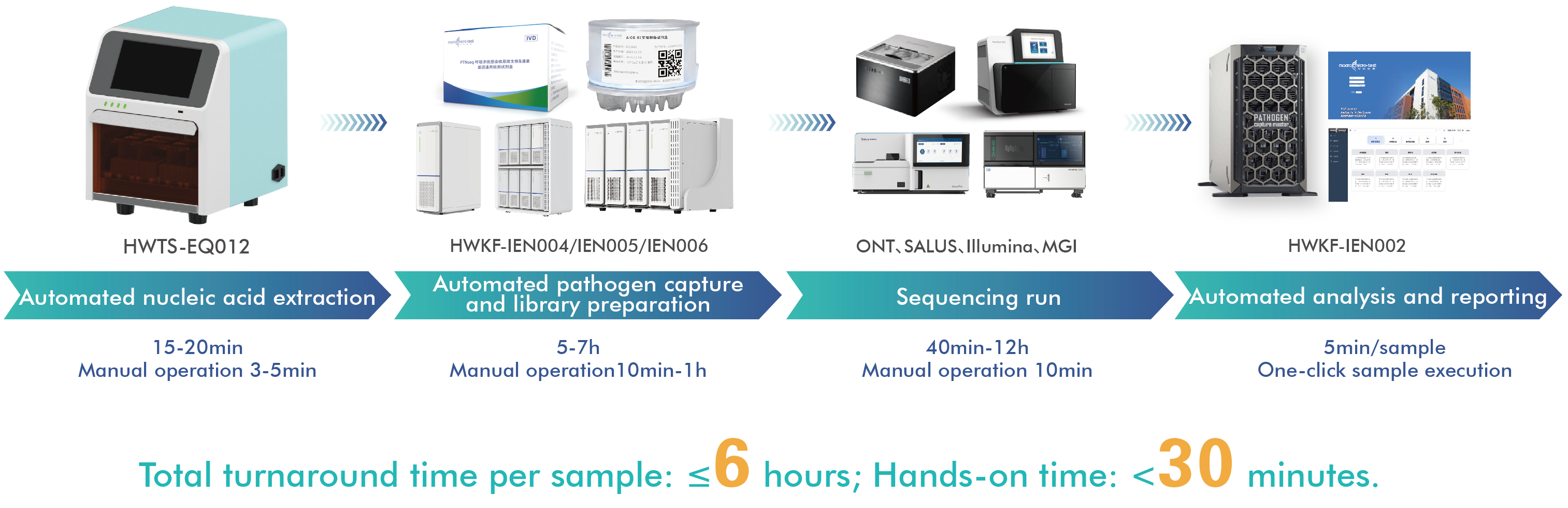
AIOS features an integrated, single-use cartridge design that consolidates nucleic acid extraction, library preparation, and purification into one fully enclosed workflow. From RNA/DNA sample input to sequencing-ready libraries, the entire process is completed automatically—minimizing hands-on time, reducing contamination risk, and ensuring reproducible results.
This “sample in, library out” capability makes AIOS especially suitable for environments where consistency, biosafety, and operational simplicity are critical.
Ready-to-Use Reagent
Regulatory Readiness Meets Proven Innovation
Prior to this approval, Macro & Micro-Test’s Fully Automated Microbial Whole-Genome Sequencing Solution had already been recognized as a Beijing New Technology & New Product. With AIOS now receiving formal
NMPA registration, the solution achieves a strong dual endorsement of innovation and regulatory compliance—reinforcing the company’s leadership in automated NGS library preparation.
Enabling Scalable Clinical and Public Health Sequencing
The approval of AIOS supports the broader transition of genomic testing from specialized laboratories to scalable, real-world clinical and public health deployment:
-For clinical laboratories:
Standardized workflows with reduced manual dependency, improved reproducibility, and faster turnaround times.
-For public health and disease control systems:
Rapid pathogen identification and large-scale screening capabilities to enhance preparedness for infectious disease outbreaks.
-For research institutions and third-party laboratories:
Lower operational complexity and training requirements, enabling stable, high-throughput data generation.
Looking Ahead
Macro & Micro-Test remains committed to delivering intelligent, efficient, safe, and compliant genomic technologies that empower precision diagnostics and public health surveillance worldwide. Building on this regulatory milestone, the company will continue to advance fully automated, integrated sequencing solutions, working closely with global partners to make genetic testing more efficient, more reliable, and more accessible.
Post time: Feb-09-2026