On January 27, Japan’s Ministry of Agriculture, Forestry and Fisheries confirmed a highly pathogenic avian influenza (HPAI) outbreak at a quail farm in Asahi City, Chiba Prefecture. This marks the 18th outbreak of the 2025-2026 avian flu season in Japan and the first for Chiba Prefecture this season.
With the culling of approximately 108,000 quails underway, movement of poultry within a 3-kilometer radius has been restricted, and the transport of birds and related products from a 3-10 kilometer zone has been prohibited.
Escalating Outbreaks
The Chiba quail farm outbreak is not an isolated incident. As of January 22, 2026, 17 avian influenza outbreaks have been reported across 12 prefectures in Japan, leading to the culling of over 4 million birds.
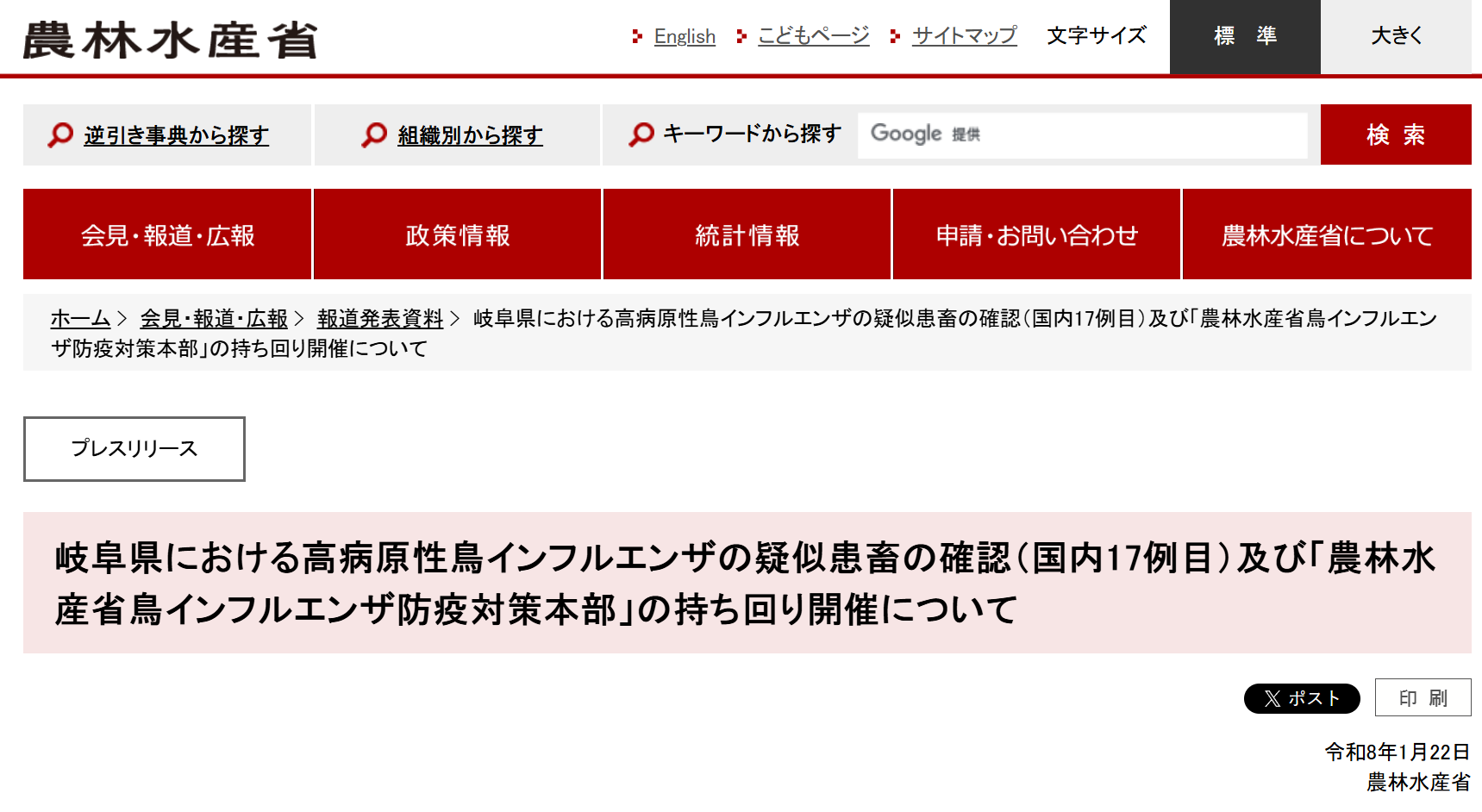
Japan is facing a persistent, multi-year avian influenza threat. From autumn 2024 to winter 2025, Japan culled approximately 9.32 million birds to control the spread, causing egg shortages and significant price increases in the market.
The threat has never been more pressing. Farm bio-security measures, migratory bird pathways, and increasing international exchanges all form potential channels for viral transmission. Each outbreak in animals serves as a test for our global public health defense systems.
A Global Surge
The threat of avian influenza has long transcended borders, intensifying into a global crisis. In Europe, Germany recently culled nearly one million birds. In the United States, 2 million egg-laying hens were destroyed due to infection, with H5N1 detected in dairy herds across multiple states.
Cambodia has reported several human H5N1 infections, including six fatalities. A significant development emerged from Washington State, USA: the first confirmed human death from the H5N5 strain. The patient was an elderly individual with pre-existing health conditions who kept a backyard flock.
While health officials stress that the public risk remains low and no human-to-human transmission has been identified, the growing risk of cross-species transmission presents a clear and escalating threat to human health.
The global distribution and spread of various influenza subtypes form a complex network, with the virus continuously circulating and mutating within animal hosts.
Precision Detection for Defense
In this race against the virus, rapid and accurate testing forms the indispensable first line of defense. This is true for clinical screening in hospitals, surveillance by public health authorities, and health checks at border controls — reliable diagnostics are crucial.
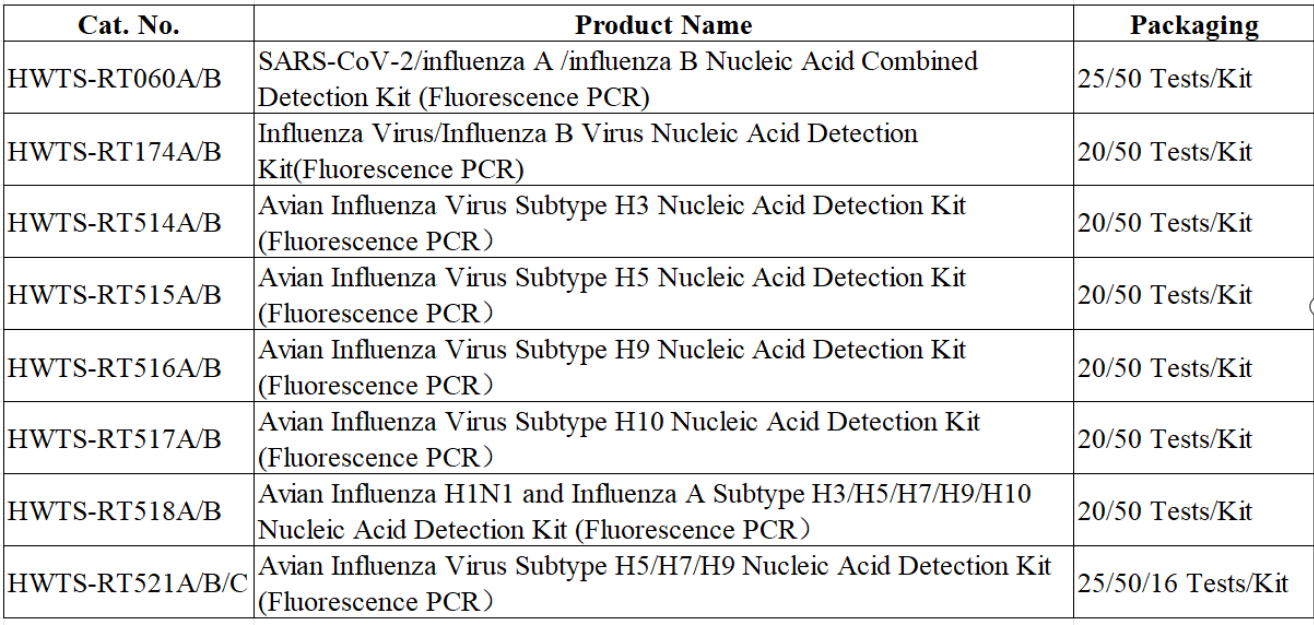
Macro & Micro-Test offers a comprehensive portfolio of fluorescent PCR detection kits for multiple influenza virus subtypes, including H1N1, H3, H5, H7, H9, and H10. This enables early detection and accurate subtyping.
Subtype-Specific Detection — Targeting High-Risk Strains
-H5 Subtype Detection Kit: Detects highly pathogenic H5 strains such as H5N1 that can infect humans. Ideal for rapid screening of suspected cases in medical facilities.
-H9 Subtype Detection Kit: Targets low-pathogenic H9 viruses occasionally found in humans. Suitable for health monitoring of high-risk populations (e.g., poultry workers, travelers), helping prevent silent transmission.
-H3/H10 Subtype Detection Kit: Designed to detect both common seasonal subtypes (H3) and rare sporadic strains (H10), filling critical gaps in influenza detection.
Multiplex Detection — Comprehensive Screening in a Single Test
-H5/H7/H9 Triple Detection Kit: Detects three major high-risk subtypes in one reaction. Perfect for large-scale screening during peak flu seasons or in densely populated areas.
-Six-Multiplex Detection Kit: Simultaneously identifies H1N1, H3, H5, H7, H9, and H10 — the ideal choice for hospitals and CDC laboratories handling complex samples (e.g., patients with unexplained fevers), minimizing the chance of missed infections.
Advanced Genomic Identification
When deeper viral analysis is required, subtyping alone is insufficient. Tracking viral mutations, tracing evolutionary pathways, and assessing vaccine strain matching demand comprehensive genomic intelligence.
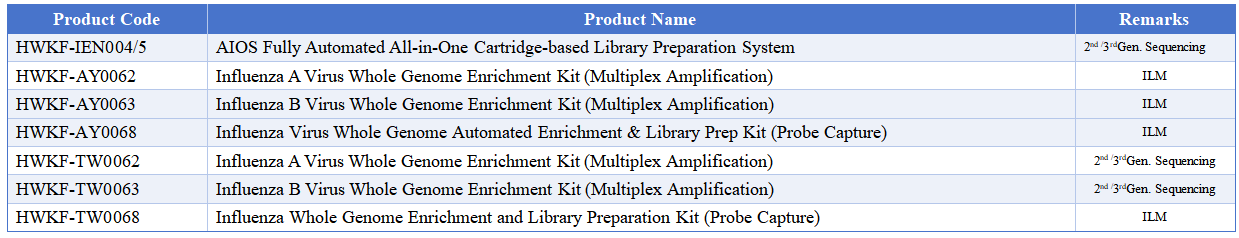
Macro & Micro-Test’s influenza whole genome sequencing solutions, utilizing high-throughput sequencing coupled with whole genome amplification, deliver complete viral genomic profiles.
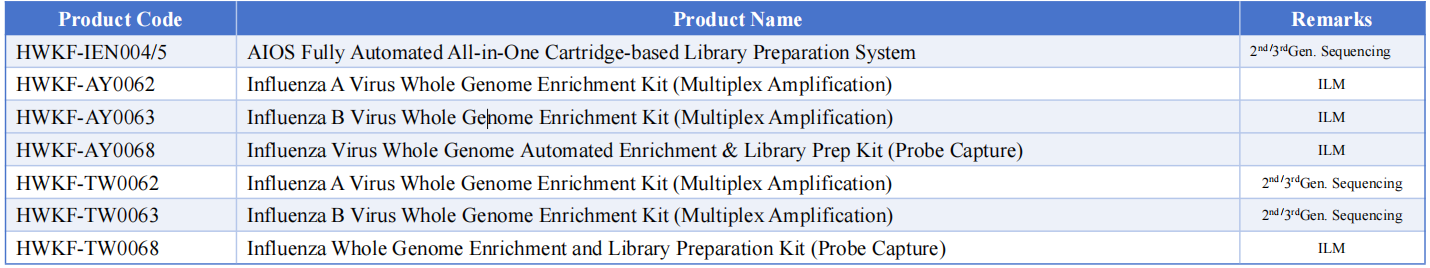
Centered on the AIOS800 fully automated library preparation system and integrated with upstream and downstream automation modules, this system creates a high-throughput, all-in-one solution for on-site deployment.
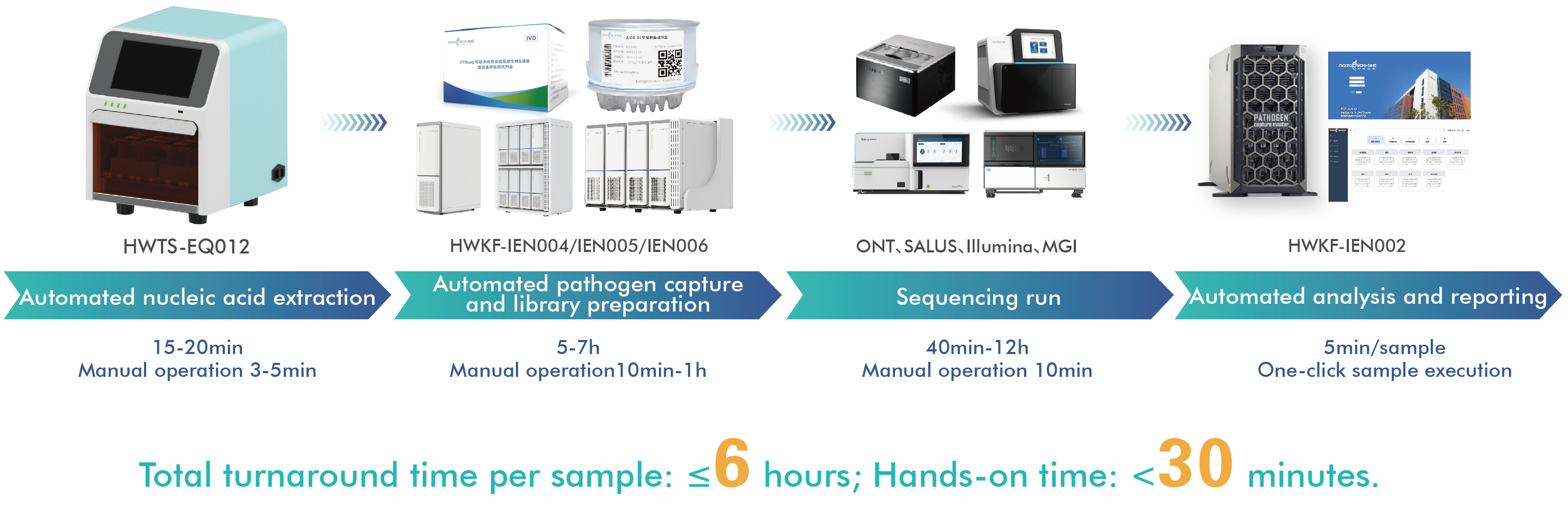
This approach meets the dual needs of influenza subtyping and resistance detection, providing comprehensive, accurate technical support for tracking viral evolution, transmission tracing, and vaccine development.
Building the Defense Network
Confronting the evolving threat of influenza viruses requires a complete diagnostic defense system covering the entire chain from rapid screening to in-depth analysis.
Hospital fever clinics and infectious disease departments can utilize these tools for accurate screening and diagnosis of influenza-like illnesses, especially potential H5N1 cases. Centers for Disease Control can leverage this technology for influenza surveillance, outbreak tracing, and contact monitoring.
From local clinics to national CDC labs, from border ports to research institutions, detection capabilities at every level constitute a critical node in the wider global biosecurity network.
Macro & Micro-Test — Precision Diagnosis for a Safer Future.
Empowering global efforts in early detection, rapid response, and effective influenza control.
Post time: Jan-28-2026