As nucleic acid testing becomes a routine necessity, have you faced these challenges: reagents that risk degradation during transport, contamination-prone opening procedures, or activity loss from repeated freeze-thaw cycles?
A century-old “age-defying” technology—vacuum freeze-drying—is now driving a revolutionary shift in the molecular diagnostics industry.
Today, let’s explore how MacroMicroTech (MMT) is tackling four major pain points with its single-use, fully formulated freeze-dried microsphere technology.
I. The Remarkable Comeback of a Century-Old Technology: A Brief History of Freeze-Drying
In 1909, scientists first used freeze-drying to preserve serum; by 1919, it enabled long-term storage of viral strains. Once hailed as the “guardian of bioactivity,” freeze-drying is now rejuvenated and widely applied in modern molecular diagnostics.
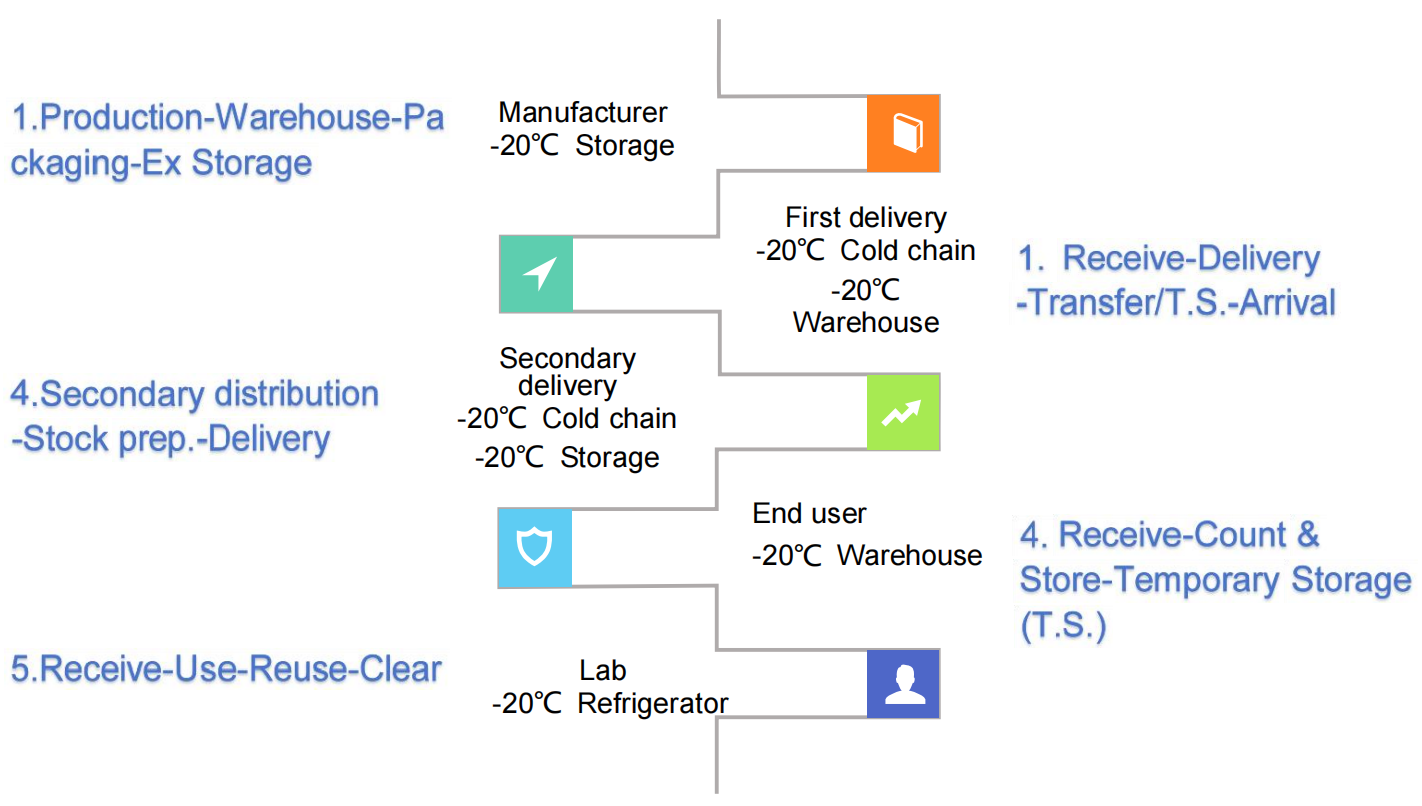
1. The Grueling Journey of Traditional Liquid Reagents
From manufacturer to end user, liquid reagents must be kept under strict cold-chain conditions throughout storage and transport. Improper handling frequently leads to enzyme degradation, probe instability, and failed reactions—a recurring issue documented in training sessions and case studies.
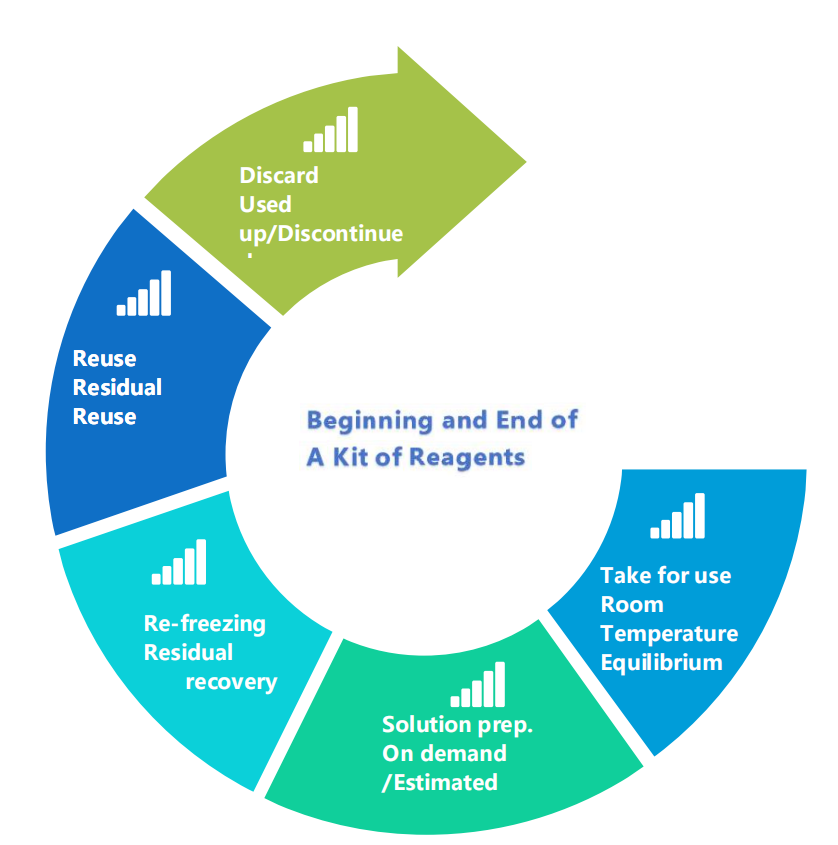
2. The High-Risk Lifecycle of Liquid Reagents
Once opened, a box of frozen reagents often endures multiple cycles of thawing, use, and re-freezing—until fully consumed or discarded—resulting in activity loss and waste.
Freeze-drying addresses these challenges at the root. By removing water under vacuum at low temperatures, enzymes and probes are “locked” in a stable state—essentially pressing pause on degradation until the moment of use.
II. Four Core Advantages: Why Freeze-Drying Is the Optimal Solution for Molecular Diagnostics
MMT’s single-use, fully formulated freeze-dried microspheres are developed to eliminate the key bottlenecks of the industry:
III. Technology Breakthrough: Why Did MMT Choose Freeze-Dried Microspheres?
Among various forms such as in-vial or PCR-tube lyophilization, MMT has pioneered a freeze-dried microsphere format that achieves three key breakthroughs:
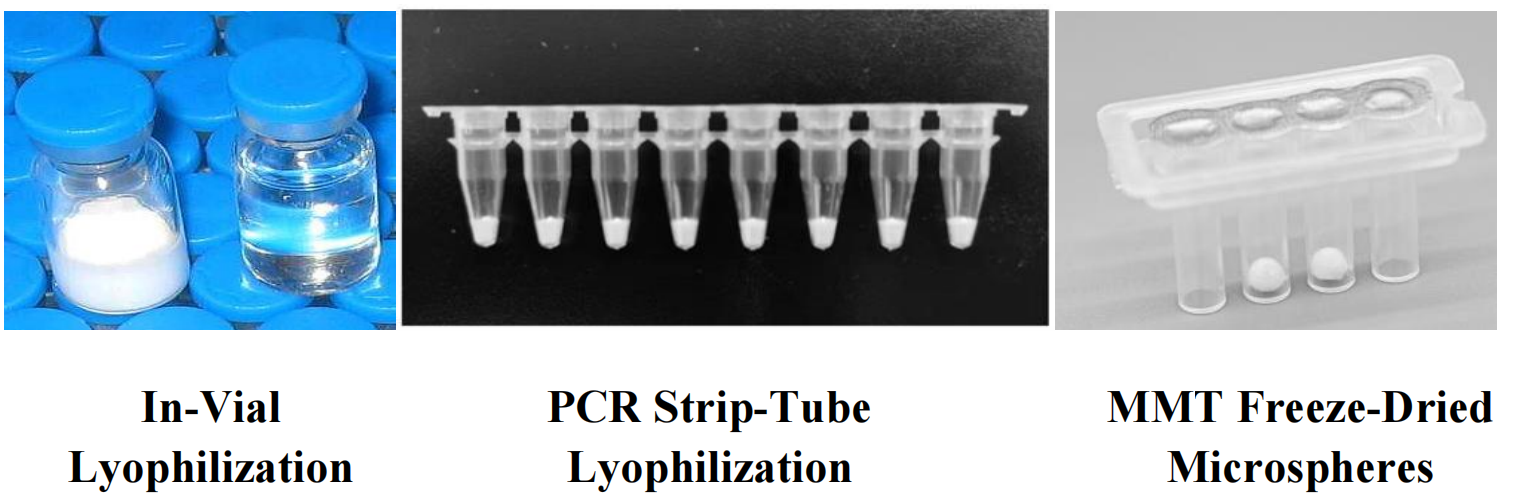
u “One Microsphere = One Complete Reaction”
Each 2 mm Microsphere contains all components
—primers, enzymes, dNTPs—ensuring accuracy and eliminating aliquoting errors.
u 1-Minute Reconstitution
Special excipients allow the microspheres to dissolve 50% faster than conventional freeze-dried formats.
u Open-Format Compatibility
Compatible with a wide range of consumables and devices (e.g., 8-strip PCR tubes, single-use formats), ensuring flexibility across applications.
IV. The Future Is Now: How Freeze-Drying Will Reshape Molecular Diagnostics
As molecular diagnostics move closer to point-of-care, home testing, and primary healthcare settings, MMT is ready with its robust freeze-drying platform.
For example, in respiratory panel testing, freeze-dried microspheres deliver reliable results with ease, without cold chain or complex setup.
On AIO800
From “cold chain anxiety” to “room temperature freedom,” from “complex procedures” to “one-step detection,” freeze-drying technology is redefining the boundaries of reliability in molecular diagnostics.
MMT will continue to innovate along the path of stability, efficiency, simplicity, and convenience—empowering precision diagnostics to break free from time and location constraints.
Post time: Dec-26-2025