Human papillomavirus (HPV) infections are extremely common. While the majority of infections are cleared by the immune system within 1-2 years without consequence, a small percentage of persistent high-risk HPV infections can silently initiate a carcinogenic process that may span 10 to 20 years. This journey typically progresses from persistent infection to precancerous lesions (CIN), and potentially to cervical cancer.
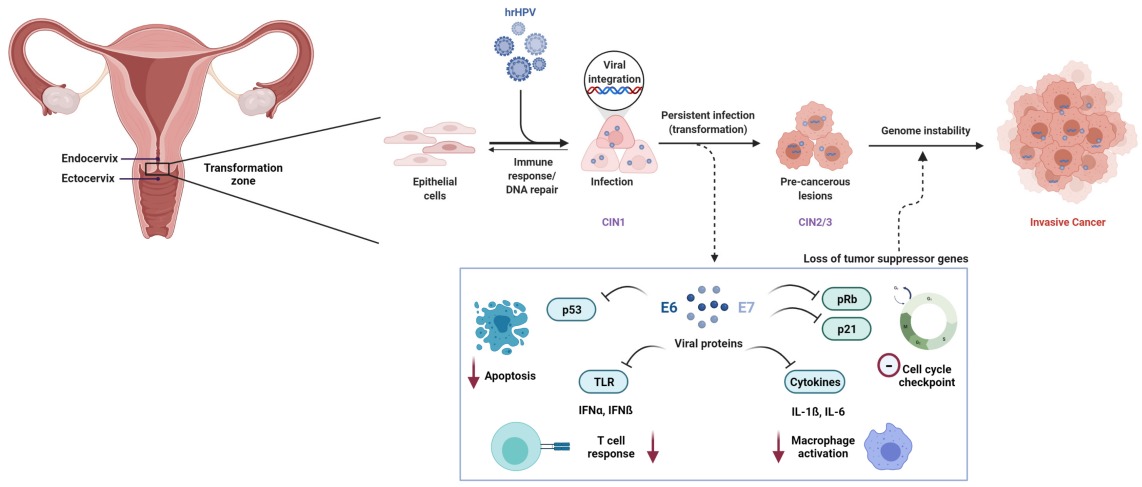
This long “window of opportunity” is where the true value of cervical cancer screening and prevention lies. The primary goal of regular screening is to identify and intervene at the precancerous stage—or even during persistent infection—to effectively prevent cancer development.
The Evolution of Screening: From “If” to “Which”
Traditional HPV screening methods generally detect the presence of “high-risk HPV (HR-HPV).” However, this approach is no longer sufficient for precision healthcare. The HPV family consists of over 200 types, with significantly different risk profiles:
High-Risk types (HR-HPV): Such as HPV 16 and 18, which are the primary causes of cervical cancer.
Low-Risk types (LR-HPV): Such as HPV 6 and 11, which primarily cause genital warts and are generally non-oncogenic.
Simply knowing that an HPV infection is present without identifying the specific type creates clinical and communication challenges:
Inaccurate Risk Stratification: The risk posed by HPV 16 is far greater than that of other high-risk types. Grouping all high-risk types together leads to imprecise risk assessment.
Unexplained Clinical Symptoms: For patients presenting with genital warts, identifying specific low-risk types (e.g., 6/11) allows for an accurate diagnosis and distinct management from high-risk infections.
Unnecessary Patient Anxiety: A positive result without genotyping may cause patients with low-risk infections to mistakenly believe they are at high risk for cancer.
Thus, HPV genotyping has become the new standard in screening. It not only confirms the infection but also identifies the type, providing crucial information for risk assessment, follow-up intervals, and clinical decision-making.
A Comprehensive and Precise Solution: The HPV 28 Genotyping Assay
Addressing these clinical needs, Macro & Micro-Test’s HPV 28 Genotyping Assay offers a comprehensive, accurate, and streamlined solution.
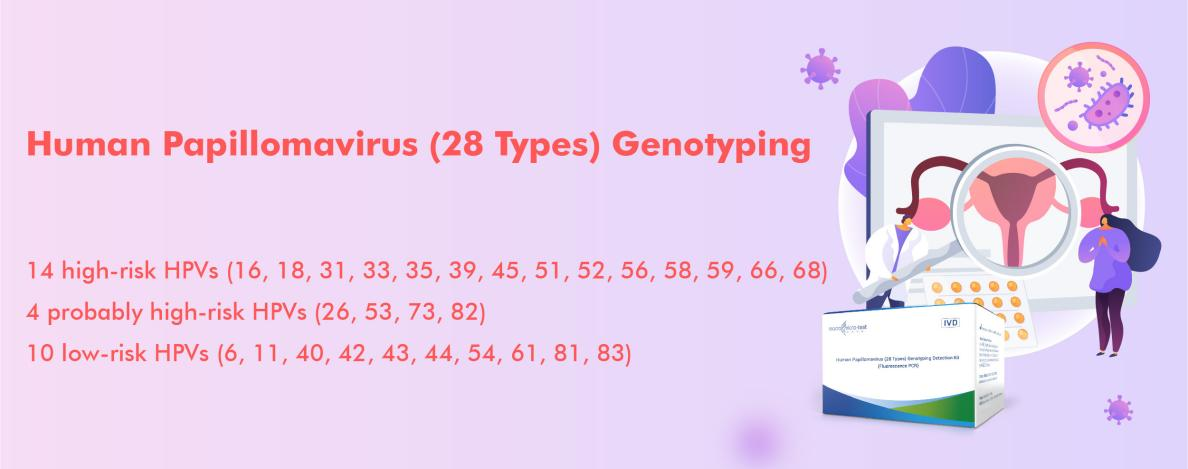
Core Value Proposition:
-Complete Spectrum Coverage: Simultaneously detects 28 HPV genotypes, including all 14 key high-risk types and 14 common low-risk types, providing a true ”one-test” solution.
-Precise Genotyping for Informed Action: Clearly distinguishes high-risk types like HPV 16/18 from others, enabling differentiated clinical management (e.g., immediate colposcopy referral or extended surveillance). It also identifies low-risk infections causing genital warts.
-Technical Excellence & Accessibility: The assay features high sensitivity and specificity. It supports urine self-sampling in addition to cervical swabs, and is available in a room-temperature stable lyophilized format, significantly enhancing screening accessibility.
-Broad PCR Compatibility: It integrates seamlessly with most mainstream PCR systems worldwide.
The path from HPV infection to cervical cancer, though long, is preventable. Prevention hinges on using precise screening tools to effectively intercept the process at every stage.
The HPV 28 Genotyping Assay is exactly such a tool. It elevates screening from a vague “risk alert” to a clear “action plan,” empowering clinicians and protecting patients. It is a critical component of building an effective cervical cancer prevention system.
For more product information or partnership opportunities, please contact: marketing@mmtest.com
Post time: Dec-24-2025
