January 2026 marks Cervical Cancer Awareness Month, a pivotal moment in the World Health Organization’s (WHO) global strategy to eliminate cervical cancer by 2030. Understanding the progression from an HPV infection to cervical cancer is crucial in empowering people to contribute to this global public health initiative.

From HPV to Cancer: A Slow Process We Can Interrupt
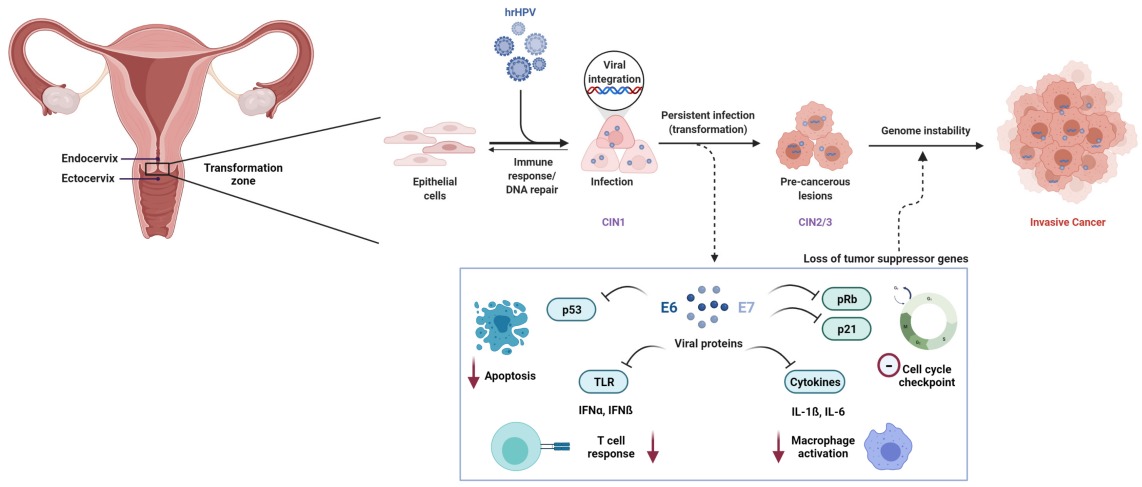
The path from a persistent high-risk HPV infection to cervical cancer is a gradual, taking 10 to 20 years. This extended timeline provides an invaluable opportunity for effective screening and prevention.
Initial HPV Infection (0–6 months):
HPV enters the cervix through micro-abrasions in the epithelial cells. In most cases, the immune system successfully clears the virus within 6 to 24 months, and there is no lasting damage.
Transient Infection (6 months to 2 years):
During this stage, the body’s immune system continues to fight off the infection. In about 90% of cases, the infection resolves without causing any complications, posing minimal risk for cervical cancer.
Persistent Infection (2–5 years):
In a small group of women, the HPV infection becomes persistent. This is when the virus continues to replicate in cervical cells, causing ongoing expression of the viral oncogenes E6 and E7. These proteins disable important tumor suppressors leading to cellular abnormalities.
Cervical Intraepithelial Neoplasia (CIN) (3–10 years):
Persistent infections can lead to precancerous changes in the cervix known as Cervical Intraepithelial Neoplasia (CIN). CIN is graded into three levels, with CIN 3 being the most severe and most likely to progress into cancer. This stage usually develops over 3 to 10 years after persistent infection, during which regular screening is essential for detecting early changes before cancer forms.
Malignant Transformation (5–20 years):
If CIN progresses without treatment, it can eventually transform into invasive cervical cancer. The process from persistent infection to full-blown cancer can take anywhere from 5 to 20 years. Throughout this long timeline, regular screening and monitoring are crucial to intervene before cancer develops.
Screening in 2026: Simpler, Smarter, and More Accessible
Global guidelines have evolved, with the most effective practice now being primary HPV testing. This method detects the virus directly and is more sensitive than traditional Pap smears.
-The Gold Standard: High-risk HPV DNA Test
Highly sensitive for detecting HR-HPV DNA, ideal for broad primary screening and early HPV infections, with a recommended interval of every 5 years for women aged 25–65.
-Follow-up Tests: Pap Smear and HPV mRNA Testing
If an HPV test is positive, a Pap smear is typically used to determine whether a colposcopy (a closer examination of the cervix) is necessary. HPV mRNA testing is an advanced method that checks whether the virus is producing cancer-related proteins, helping doctors identify which infections are more likely to lead to cancer.
When to Get Screened (Based on Major Guidelines):
-Start regular screening at age 25 or 30.
-If your HPV test is negative: Repeat screening in 5 years.
-If your HPV test is positive: Follow your doctor’s advice, which may involve a Pap smear or retesting in 1 year.
-Screening may stop after age 65 if you have a consistent history of normal results.
The Future is Here: Tech Making Screening Easier and More Precise
To meet the WHO’s 2030 elimination targets, screening technology is advancing rapidly to address barriers like accessibility, complexity, and accuracy. Modern systems are designed to be highly sensitive, user-friendly, and adaptable to any settings.
Macro & Micro-Test’s AIO800 Fully Automated Molecular System with the HPV14 Genotyping Kit is th next-generation approach crucial for large-scale screening:

WHO-Aligned Precision: The kit detects and differentiates all 14 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68), in line with global prevention protocols, ensuring identification of the strains most linked to cervical cancer.
-Ultra-Sensitive, Early Detection: With a detection threshold of just 300 copies/mL, this system can detect early-stage infections, ensuring that no risks are overlooked.
-Flexible Sampling for Better Access: Supporting both clinician-collected cervical swabs and self-collected urine samples, this system dramatically improves accessibility. It offers a private, convenient option that can reach under-served communities.
-Built for Real-World Challenges: The solution features dual reagent formats (liquid and lyophilized) to overcome cold-chain storage and transport hurdles.
-Wide Compatibility:It’s compatible with both the AIO800 automated POCT for Sample-to-Answer operation and mainstream PCR instruments, making it adaptable for labs of all sizes.
-Reliable Automation: The fully automated workflow minimizes manual intervention and human error. Combined with an 11-layer contamination control system, it ensures consistently accurate results—critical for effective screening.
The Path to Elimination by 2030
We have the tools we need to reach the WHO’s “90-70-90” strategy for cervical cancer elimination by 2030:
-90% of girls fully vaccinated against HPV by age 15
-70% of women screened with a high-performance test by ages 35 and 45
-90% of women with cervical disease receiving treatment
Technological innovations that improve sensitivity, accessibility, and operational simplicity will be key to achieving the second “70%” screening target globally.
What YOU Can Do
Get Screened: Talk to your doctor about the appropriate test and schedule for you. Ask about available testing options.
Get Vaccinated: HPV vaccination is safe, effective, and recommended for adolescents and young adults. Inquire about catch-up doses if you’re eligible.
Know the Signs: Seek medical advice if you experience unexpected bleeding, especially after sex.

The long timeline from HPV to cancer is our greatest advantage. Through vaccination, advanced screening, and timely treatment, cervical cancer elimination is an achievable global goal.
Contact us: marketing@mmtest.com
Post time: Jan-15-2026